LeoPARDS

Levosimendan for the Prevention of Acute oRgan Dysfunction in Sepsis
Gordon NEJM 2017; October 5. DOI: 10.1056/NEJMoa1609409
Clinical Question
- In adult patients who have sepsis, does levosimendan reduce the incidence and severity of acute organ dysfunction compared with placebo?
Design
- Randomised, double-blind, placebo-controlled multi-centre trial
- Randomisation by computer generated number and stratified by ICU on a 1:1 basis in permuted blocks
- Intention to treat analysis
- Enrollment, randomisation and data collection were performed by means of an online data collection tool
- A calculated sample of 500 patients provided 90% power to detect a difference of 0.5 points in the mean SOFA score, assuming a standard deviation of 1.5 and a significance level of 0.05. To allow for a 3% rate of withdrawal of consent, the recruitment target was 516 patients
Setting
- 34 general ICUs in the UK
- Jan 2014 – Dec 2015
Population
- Inclusion: Adult patients with suspected or confirmed infection and 2 or more SIRS criteria who are dependent on vasopressors for at least four hours to maintain their blood pressure
SIRS criteria are: fever (>38C) or hypothermia (< 36C), HR > 90 beats/minute), RR > 20 breaths/minute or PaCO2 < 4.3 kPa) or need for mechanical ventilation, abnormal leukocyte count (> 12,000 cells/mm3, < 4000 cells/mm3, or > 10% immature forms)
- Exclusion: More than 24 hours since meeting all the inclusion criteria; end-stage renal failure at presentation (previously dialysis-dependent); severe hepatic impairment (Child-Pugh class C); a history of Torsades de Pointes; known significant mechanical obstructions affecting ventricular filling or outflow or both; treatment limitation decision in place (eg DNAR or not for ventilation/ dialysis); known or estimated weight >135kg; pregnancy; previous treatment with levosimendan within 30 days; known hypersensitivity to levosimendan or any of the excipients; known to have received another investigational medicinal product within 30 days or currently in another interventional trial that might interact with the study drug
- 2382 patients were assessed for eligibility; 516 patients were recruited
- Baseline characteristics were similar in both groups:
- median time to recruitment was 16 hours after the initiation of vasopressors
- median dose of norepinephrine was 0.28 μg/kg/min at the time of starting the study drug
- more patients in the levosimendan group had ischaemic heart disease (17.8% vs 12.1%)
Intervention
- Intravenous infusion of levosimendan 0.05 to 0.20 μg/kg/min for 24 hours in addition to standard care
- The study drug infusion was commenced at 0.1 µg/kg/min, and if tolerated, increased after 2-4 hours to 0.2 µg/kg/min for the remaining 20-22 hours (maximum 24 hour infusion)
- If there were any subsequent adverse effects (hypotension and/or tachycardia), the rate of infusion was reduced back to 0.1 µg/kg/min
- If there were adverse effects at an infusion rate of 0.1 µg/kg/min (either initially or later) then the rate of infusion was be reduced to 0.05 µg/kg/min or discontinued
Control
- Intravenous infusion of placebo for 24 hours in addition to standard care
In both groups
- The study drug was not be started until the treating physician was confident that adequate fluid resuscitation has been achieved and the patient has reached their target mean arterial pressure (suggested target 65-70 mmHg)
- Examples of appropriate targets for adequate fluid resuscitation included any or all of the following:
- Central venous pressure ≥8mmHg (≥12 mmHg in mechanically ventilated patients); good peripheral perfusion on clinical examination; other measures of cardiac output / flow, e.g. Stroke Volume Variability (SVV) and Global End-Diastolic Volume Index (GEDVI)
- The lowest dose of vasopressors that maintained tissue perfusion and the target mean arterial pressure was recommended
- Additional inotropic agents (dobutamine recommended) could be used if clinically indicated (e.g for low cardiac output state after fluid resuscitation), with lowering of the dose once adequate oxygen delivery had been achieved
Outcome
- Primary outcome: mean daily Sequential Organ Failure Assessment (SOFA) score in the ICU up to day 28
- no significant difference in the mean (±SD) SOFA score between the levosimendan group and the placebo group (6.68±3.96 vs. 6.06±3.89; mean difference, 0.61; 95% confidence interval [CI], −0.07 to 1.29; P=0.053)
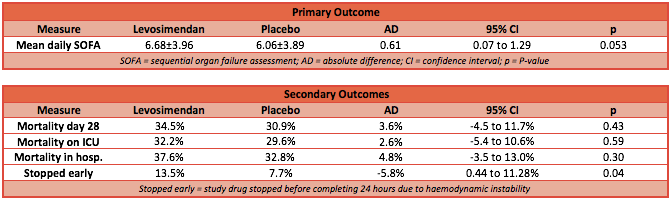 table corrected post first publishing
table corrected post first publishing
- Secondary outcome:
- Death at 28 days, at ICU discharge and at hospital discharge: no statistical difference between patients in the levosimendan and placebo group
- 28 day mortality: 34.5% vs 30.9% P=0.43
- Mortality at ICU discharge: 32.2% vs 29.6% P=0.59
- Mortality at hospital discharge: 37.6% vs 32.8% P=0.3
- cardiovascular effects:
- Study drug infusion was stopped before before the 24 hour time point owing to haemodynamic instability: 13.5% in the levosimendan group vs 7.7% in the placebo group
- mean arterial pressure was lower in those patients in the levosimendan group in the first 24 hours
- the rate and duration of norepinephrine infusion were higher on the levosimendan group
- the heart rate over the first 4 days was significantly higher in patients in the levosimendan group
- time to extubation
- patients in the levosimendan group were less likely than those in the placebo group to be successfully weaned from mechanical ventilation over the period of 28 days (hazard ratio, 0.77; 95% CI, 0.60 to 0.97; P=0.03)
- No statistical difference in any of the following secondary outcomes
- median number of catecholamine free days
- median number of ventilation free days
- incidence & duration of renal failure (AKIN criteria)
- median length of ICU and hospital stay
- Adverse events
- more patients in the levosimendan group than in the placebo group had supraventricular tachyarrhythmia: 3.1% vs 0.4%; P=0.04
- no statistical difference in any other life threatening arrhythmia, myocardial infarction or acute coronary syndrome
- Death at 28 days, at ICU discharge and at hospital discharge: no statistical difference between patients in the levosimendan and placebo group
Authors’ Conclusions
- The addition of levosimendan to standard treatment in adults with sepsis was not associated with less severe organ dysfunction or lower mortality. Levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia
Strengths
- Randomisation sequence was prepared by an independent statistician
- Trial specific labelling and packaging was used for the study drugs
- Patients and clinical and research staff remained unaware of the trial group assignment
- Subgroup analyses were planned a priori
Weaknesses
- No echocardiographic analyses were performed
- The target mean arterial pressure was frequently exceeded in both groups
- The primary outcome was an average of the SOFA scores from each patient’s complete stay on ICU. This is arguably a surrogate for mortality and other meaningful patient-centred outcomes. Also, there may be a difference in the distribution of SOFA scores against time, but by averaging the scores this difference has been lost in the analysis.
The Bottom Line
- In adults with sepsis, a 24 hour infusion of levosimendan, in addition to standard treatment, was not associated with less severe organ dysfunction or mortality compared with placebo and standard treatment. There may be associated harm from levosimendan given the higher incidence of haemodynamic instability and the longer duration of mechanical ventilation.
External Links
- [article] Levosimendan for the Prevention of Acute oRgan Dysfunction in Sepsis
- [further reading] LeoPARDS protocol
- [videocast] Interview with Professor Anthony Gordon (iCTV Hot Topics ESICM)
- [review] Levosimendan in the Treatment of Acute Heart Failure, Cardiogenic and Septic Shock: A Critical Review. JICS
- [blog] LEOPARDS…EVEN MORE DANGEROUS WHEN YOU’RE SEPTIC! Critical Care Northampton
Metadata
Summary author: Steve Mathieu
Summary date: 6th October 2017
Peer-review editor: Duncan Chambler



The quest for magic bullet in treatment of septic shock is driving us to test therapies in large RCT which do not have good enough data prior to support the case.This raises a wider debate of conducting a large RCT based on prior flimsy data .
“The study drug was not be started until the treating physician was confident that adequate fluid resuscitation has been achieved and the patient has reached their target mean arterial pressure (suggested target 65-70 mmHg)”
By my experience this is NOT the time when I consider starting treatment with levosimendan. The purpose of the drug for me would be to achieve better organ perfusion and one of the goals would be to achieve target MAP by using levosimendan, not infusing it after MAP-target already been achieved.
Pingback: Länkar v45 | Internmedicin
Pingback: LEVO-CTS – The Bottom Line
Pingback: CHEETAH – The Bottom Line
Seems a ridiculous thing to study, before any proven benefit in other potentially higher yield areas (cardiac patients). Putting patients at risk unnecessarily and wasting valuable research time and money.