PAUSE
Perioperative Management of Patients with Atrial Fibrillation Receiving a Direct Oral Anticoagulant
Douketis, JD. JAMA Internal Medicine; Published online August 5 2019. doi:10.1001/jamainternmed.2019.2431
Clinical Question
- In patients with atrial fibrillation (AF) on direct oral anticoagulant (DOAC) medications, does a standard approach to therapy interruption and resumption in the perioperative period result in an acceptable risk for bleeding and thromboembolic events?
Background
- The perioperative management of DOAC medications in patients with AF undergoing surgery varies widely in clinical practice
- There is a balance between avoiding thromboembolic complications of AF and bleeding due to the surgery
- It is estimated that 6 million patients worldwide with AF on DOACs will have surgery each year
- The rate of 30 day bleeding and thromboembolism events is known with warfarin interruption and resumption, so this was the comparator in this study
Design
- A prospective cohort study: the PAUSE study (Perioperative Anticoagulation Use for Surgery Evaluation)
- It was not felt to be ethical to conduct a randomised controlled study. For example, a trial with longer periods off the DOACs did not make sense from a pharmacological perspective and would expose the patient to excess risk
- The cohort design is a good technique to assess an outcome when the rates of the clinical outcomes are low
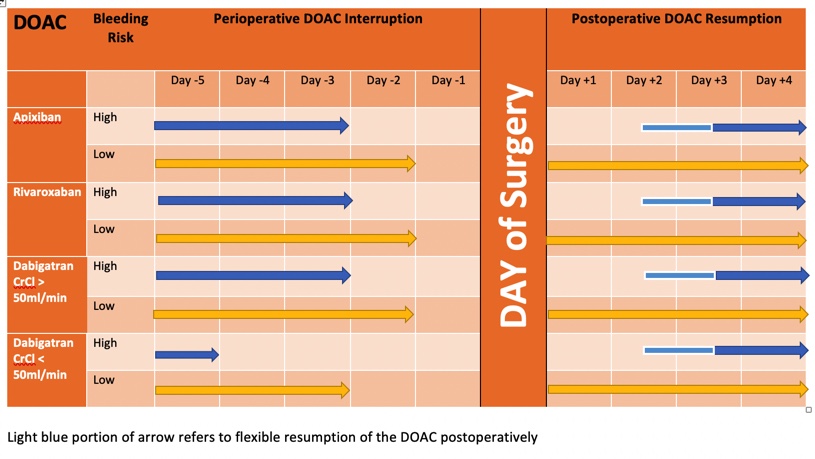
- Surgery was assessed as having a low bleeding or high bleeding risk
- The timing of the interruption of the DOAC dosing prior to surgery was determined by the bleeding risk and in the case of Dabigatran, whether the patient had renal impairment
- The timing of the resumption of the DOAC dosing after surgery was determined by the bleeding risk
- The sample size of 987 per DOAC group provided 80% power at 0.05 significance to detect a rate of major bleeding <2% and rate of arterial thromboembolic <1.5%
Setting
- 23 clinical centres in Canada, USA and Europe enrolled patients
- Enrolment dates: August 1 2014-July 31 2018
Population
- Inclusion: Patients on DOAC for AF having elective surgery
- Exclusion: Impaired renal function: CrCl<25ml/min for apixaban, <30ml/min for Dabigatran users, cognitive impairment, psychiatric illness, those who did not consent, previous study participation
- 3007 patients for the primary analysis: 1257 apixiban, 1082 rivaroxaban, 668 Dabigatran. Baseline mean CHADS2-VA2Scb score and HAS-BLED scores were similar between the DOAC groups and similar to population-based studies in patients with AF
- Approximately 1/3 of patients were classified as having high bleeding risk surgery, 2/3 as low bleeding risk surgery
- 87% adhered to protocol and were analysed in the secondary analysis (as far as adhering to preoperative interruption and postoperative resumption)
- Low Bleeding Risk surgery
- Gastrointestinal procedure (eg colonoscopy)
- Cardiac procedure (eg PPM insertion)
- Dental procedure (eg tooth extraction)
- Skin procedure (eg skin biopsy)
- Eye procedure (eg cataract surgery)
- High Bleeding Risk surgery: there was some flexibility with resumption of DOAC
- Any surgery with neuraxial anaesthesia (eg epidural)
- Major intracranial/neuraxial surgery (eg brain cancer resection)
- Major thoracic (eg pneumonectomy), vascular (eg CEA), abdominopelvic (eg pancreatic cancer resection), orthopaedic (eg knee arthroplasty), cardiac surgery (eg CAGs)
- Other major cancer or reconstructive surgery
Intervention
- The DOAC was interrupted 1 day before low bleeding risk surgery and 2 days before high bleeding risk surgery (Dabigatran earlier if renal impairment)
- The DOAC was resumed 1 day after low bleeding risk surgery and 2-3 days after high bleeding risk surgery
- Patients at high risk for venous thromboembolism could receive a prophylactic dose of heparin after the operation until DOAC therapy resumption
- The patients did not receive any bridging anticoagulation
Control
- There was no control group in this observational cohort study
- Periprocedural bleeding and thromboembolic events have previously been studied in patients who have interrupted warfarin for surgical procedures and these figures were used as a comparator. This allowed calculation of the sample size
Outcome
- Primary outcomes: Major bleeding and arterial thromboembolic complication (CVA, TIA, systemic embolisation) – assessed from the time of interruption of DOAC until Day 30 after the operation
- Major Bleeding Rates at Day 30:
- Apixaban 1.35% (95% CI 0-2%)
- Dabigatran 0.9% (95% CI 0-1.73%)
- Rivaroxaban 1.85% (95% CI 0-2.65%)
- All major bleeding occurred post-operatively with median time being 2 days
- In the high bleeding risk group, rates of bleeding were 2.96% (apixaban), 0.88% (Dabigatran), 2.95% (rivaroxaban)
- Arterial thromboembolism
- Apixaban 0.16% (95% CI 0-0.48%)
- Dabigatran 0.6% (95% CI 0-1.33%)
- Rivaroxaban 0.37% (95% CI 0-0.82%)
- 9 of 10 thromboembolic events occurred post-operatively with median time 2 days
- Major Bleeding Rates at Day 30:
- Secondary outcomes:
- Clinically relevant non major bleeding: 2%
- Death 0.3%
- Myocardial infarction: 0.03%
- DVT: 0.1%
- PE: 0.2%
- Catheter associated thrombosis: 0.07% (both arterial)
- Major bleeding in patients adhering strictly to the interruption and resumption of the DOAC as per protocol
- Apixiban 1.2%, Dabigatran 1.0%, Rivaroxaban 1.69%
- Arterial thromboembolism in patients adhering strictly to the interruption and resumption of the DOAC as per protocol
- Apixiban 0.19%, Dabigatran 0.5%, Rivaroxaban 0.42%
- Residual anticoagulant level just before the procedure (anti-factor Xa assays for apixaban and rivaroxaban and dilute thrombin time for Dabigatran): The proportion with a level < 50ng/ml was 90.5% apixaban group, 95.1% Dabigatran, 96.8% rivaroxaban group
Authors’ Conclusions
- A simple standard protocol for interruption and resumption of DOACs before and after elective surgery seems to result in low rates of major bleeding and thromboembolism
Strengths
- The risk factor for thromboembolism in this cohort as measured by CHADS2-VA2Scb was similar to that in patients with AF in population based studies.
- The detection of thromboembolism and bleeding was measured out to 30 days which would be ample time to detect a complication
- The prospective cohort design is a useful study design to pick up events which are relatively rare
- Assessors of the outcomes were not aware of which DOAC the patient was on, what was the risk of bleeding from the procedure and the preoperative residual DOAC level
Weaknesses
- A cohort study may introduce selection bias. In this study 83% of patients screened were included in the study. Those who were excluded mainly did not provide consent. There may have been a reason for that this is not apparent, eg previous bleeding after surgery, which meant the patient was unwilling to consent
- Followup occurred by scheduled weekly telephone call and additional clinic visits as required. It is possible that outcomes could be missed with this approach
- Were the study patients similar to the patients in my practice? In the ICU setting patients are generally there for surgeries that would be classified as high bleeding risk. In this paper only a third of patients included were high bleeding risk surgeries, with the majority of these being cardiac surgeries. Only 47 patients (of the total cohort) had neurosurgery for instance. I would not confidently be able to apply these results to this group of high risk patient
- The authors have not provided information on baseline risk, ie what is the risk of having major bleeding after these surgeries in patients who are not on DOACs. Therefore it is difficult to assess the incremental risk for the patients who resume these agents in the post-operative situation
The Bottom Line
- This study reassures me that stopping DOACs for 1 day prior to surgery and resuming 1-3 days after surgery is safe in most patients
- However, in ICU patients, I would apply extra caution and there is still a lack of evidence in this group
- This cohort study does not help this decision making in my patient group
- I am happy that DOACs can safely be stopped 1 day prior to surgery without bridging heparin
- I will continue to liaise with surgical colleagues and use other clinical factors to decide when DOACs should be resumed after surgery
External Links
- Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant
- JAMA evidence: user guides to medical literature. Chapter 14 Harm (Observational Studies)
Metadata
Summary author: Celia Bradford @celiabradford
Summary date: Aug 30 2019
Peer-review editor: @davidslessor



