Classic Trial

Restriction of Intravenous Fluid in ICU Patients with Septic Shock
Meyhoff T. NEJM 2022; DOI: 10.1056/NEJMoa2202707
Clinical Question
- In adult patients in ICU with septic shock, does a restrictive approach to fluid therapy compared to a standard care result in fewer deaths at day 90?
Background
- Fluid therapy in the critically unwell has been widely studied across many patient populations
- Higher volumes of fluid in patients with sepsis and septic shock has been associated with harm, although a meta-analysis showed that the quality of evidence was very low
- The ANDROMEDA SHOCK trial demonstrated in those with septic shock, that by 2 hours only ~25% of patients were deemed to still be fluid responsive
- The CLASSIC pilot study showed that a restrictive fluid strategy was feasible and showed significantly less fluid was used in the first 5 ICU days in the restrictive group
- Mortality at day 90 was an exploratory outcome and this was reduced in the restrictive group, although not significantly
Design
- Stratified, parallel-group, open-label, international, randomised controlled trial
- Randomised 1:1 using a central web based system
- Block sizes of 6 or 8
- Stratified according to site and presence/absence of metastatic or haematological malignancy
- Treatment group assignments not masked for clinicians, patients or investigators
- Those on data safety monitoring committee, statisticians assessing the outcomes, and the management committee writing the abstract draft were blinded
- Received assigned intervention from randomisation until ICU discharge (for a maximum of 90 days)
- If re-admitted within 90 days then treatment assignment continued
- Data collected from medical records and registries
- Patients or relatives contacted if further information needed
- Pre-specified subgroups included need for respiratory support, AKI, Lactate > 4 mmol/L, weight > 76kg, > 30mls/kg of fluids administered in 24 hours prior to randomisation
- Power calculation
- 1554 patients needed to show a 7% absolute reduction in 90 day mortality from a baseline of 45%
- Type I and II error rates of 5% and 20% respectively
- Based on pilot study, prior RCTs and systematic reviews
- Registered with clinicaltrials.gov
Setting
- 31 ICUs in Europe
- November 2018 to November 2021
Population
- Inclusion:
- Age ≥ 18
- Septic Shock according to Sepsis-3
- Suspected or confirmed infection, plasma lactate > 2mmol/L and vasopressor requirement
- 1L of fluid administered in the prior 24 hours
- Within 12 hours of screening
- Exclusion:
- Septic shock > 12 hours
- Life threatening bleeding
- Burns > 10% TBSA
- Known Pregnancy
- 2223 screened –> 1554 randomised
- 770 to restrictive group
- 784 to standard group
- Comparing baseline characteristics of restrictive vs. standard group
- Age (yrs): 71 vs 70
- Male (%): 59.9 vs 58.2
- Median time from ICU admission to randomisation (hrs): 3 vs 3
- Median predicted mortality (%): 40 vs 40
- Source of admission (%)
- ED: 39.3 vs 38.5
- Wards: 34.2 vs 38.7
- Source of infection (%):
- GI: 36.8 vs 38.3
- Respiratory: 27.7 vs 26.5
- Urinary: 15.8 vs 17.1
- Median highest lactate (mmol/L): 3.8 vs 3.9
- Median highest dose of noradrenaline (μg/kg/min): 0.25 vs 0.23
- Median volume of IV fluids in prior 24 hours (L): 3.2 vs 3.0
- Systemic glucocorticoid use (%): 28.6 vs 29.1
- Use of respiratory support (NIV, IMV): 52.6 vs 48.6
Intervention
- Restrictive Group
- Fluids could be given for:
- Severe hypoperfusion:
- Lactate > 4 mmol/L
- MAP < 50 mmHg (with or without vasopressors/inotrope)
- Mottling beyond kneecap
- Urine Output < 0.1ml/kg (only within first 2 hours post randomisation)
- If met any of these criteria then 250-500mls bolus of crystalloid could be given followed by re-evaluation
- Overt losses (e.g. D+V) but only to correct for loss and not more
- If oral or enteral route contraindicated for water or electrolyte solutions then IV fluids could be given to correct electrolyte derangements and to ensure a 1L/24hrs total intake
- Fluids with medications and nutrition count as input
- Fluids for medications should be reduced to lowest possible volume
- Severe hypoperfusion:
Control
- Standard Care
- No upper limit for use of fluids
- IV fluids should be given based on SSC guidelines in case of circulatory impairment
- IV fluids should be given to replace observed or expected losses to correct dehydration or electrolyte derangements
- IV fluids should be given as maintenance in places with protocols that recommend this
Management common to both groups
- Isotonic crystalloids used
- Albumin only allowed following abdominal paracentesis
- Concomitant interventions for septic shock detailed in trial protocol (appropriate antibiotics, noradrenaline as a vasopressor, source control and RRT for routine indications)
- All other treatment including diuretic use at discretion of treating clinicians
Outcome
- Primary outcome:
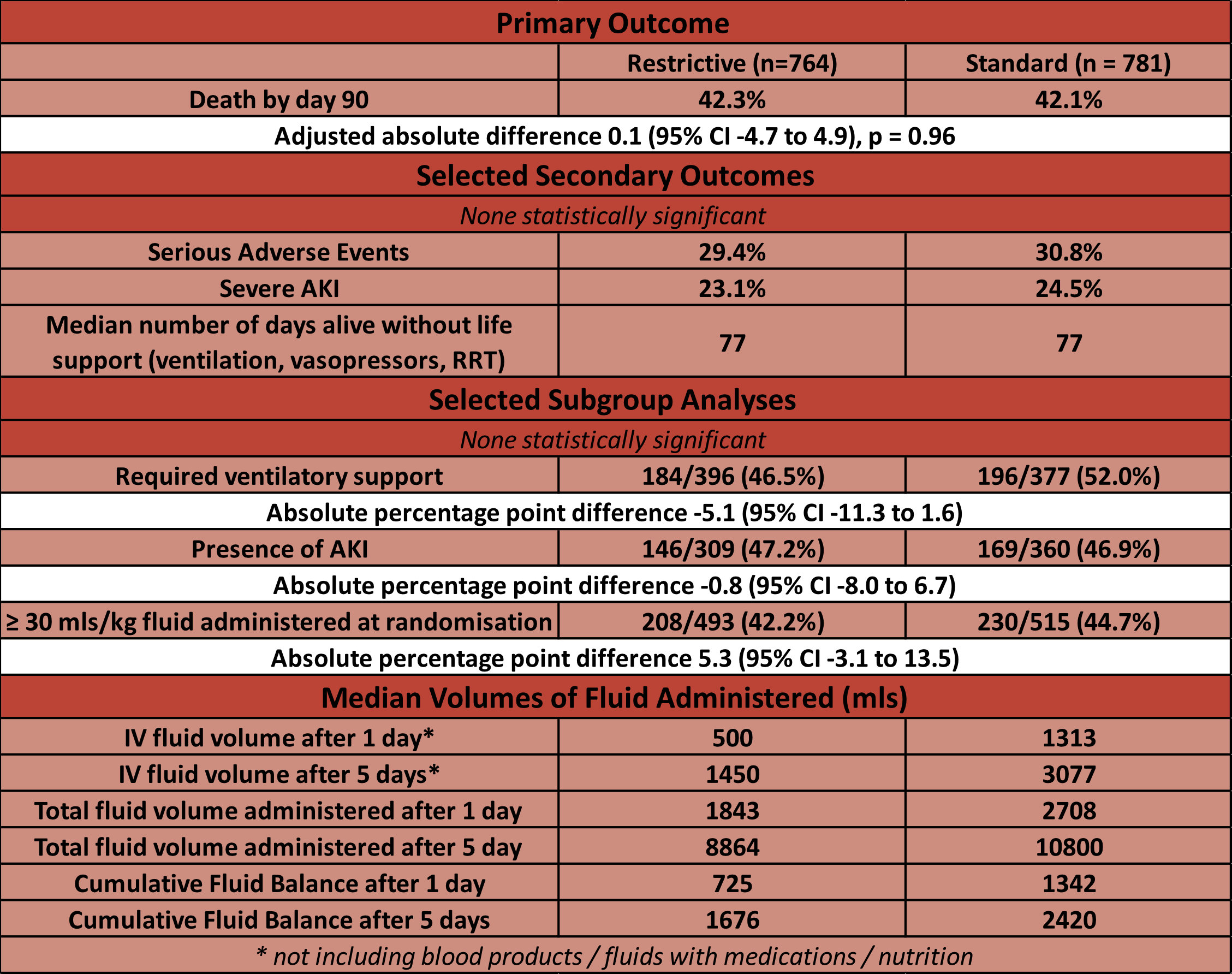
- Death by day 90
- Restrictive 42.3% vs Standard 42.1%
- Adjusted absolute difference 0.1 (95% CI -4.7 to 4.9), p = 0.96
- Secondary outcomes:
- No significant difference in:
- Number of Serious Adverse Events
- Number of days alive without life support
- Number of days alive and out of the hospital
- No significant difference in:
Authors’ Conclusions
- In adult ICU patients with septic shock, IV fluid restriction did not result in fewer deaths at 90 days compared to standard IV fluid therapy
Strengths
- Randomised
- Multi-centre trial involving 31 ICUs across 8 European countries increases external validity
- Pre-published statistical analysis plan
- Intention to treat analysis
- Minimal loss to follow up (0.6%)
- Balanced baseline characteristics
- Unwell patient cohort
- Achieved a separation in fluids administered via trial protocol
Weaknesses
- Unblinded
- The most common infection source was GI – this may have necessitated large volumes of replacement fluid if diarrhoea or vomiting were prominent features
- Powered for a 7% reduction in mortality
- Large volumes of fluid given outside of the trial protocol
- Nearly 3L of fluid given to each group in 24 hours prior to randomisation
- The total difference in fluids administered by day 5 was only ~2L (8.8L vs 10.8L), with a difference of only ~800mls in the cumulative day 5 fluid balance
- These similarities between the groups may result if the standard care delivered in ICUs is already a relatively restrictive (e.g. no maintenance) fluid strategy
- This potentially biases the results towards the null
- Large numbers of protocol violations (% patients with at least one violation)
- 21.5% in restrictive group vs 13.0% in standard group
- In restrictive group 64% of violations were for fluid bolus use without criteria being met
- Violations resulted in median of 97ml fluid administered/day
- In standard group 100% of violations were for no IV fluid being given on any single day in ICU
- This was 13% of all patients in the standard group
- In restrictive group 64% of violations were for fluid bolus use without criteria being met
- However, there was no difference in outcome when violations removed in per-protocol analysis
- 21.5% in restrictive group vs 13.0% in standard group
The Bottom Line
- This study did not confirm my pre-existing biases, in that a restrictive fluid strategy was not shown to be superior, with respect to 90 day mortality, to standard fluid management currently being practiced in European ICUs
- However, the 5 day cumulative fluid balance in the standard group was not much greater than the restrictive group. Therefore care is still required to ensure that every patient in ICU has a vigilant and considered fluid management strategy. This includes the de-escalation or evacuation phase of fluid therapy.
External Links
- article Restriction of Intravenous Fluids in ICU patients with Septic Shock
- further reading editorial
Metadata
Summary author: George Walker @hgmwalker
Summary date: 17th June 2022
Peer-review editor: @davidslessor
Picture by: Pixabay/Pexels




Describes how the Classic Trial fits into the field and is relevant to it. It is a valuable resource for understanding key findings and their implications for clinical practice and patient care