WOMAN Trial
The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial
WOMAN Trial Collaborators. Lancet 2017; Published Online April 26, 2017 http://dx.doi.org/10.1016/ S0140-6736(17)30638-4
Clinical Question
- Does the early administration of tranexamic acid (TXA), compared with placebo, reduce death from bleeding in women with post-partum haemorrhage (PPH)?
Background
- PPH (blood loss > 500mls within 24 hours of giving birth) is the leading cause of maternal death worldwide
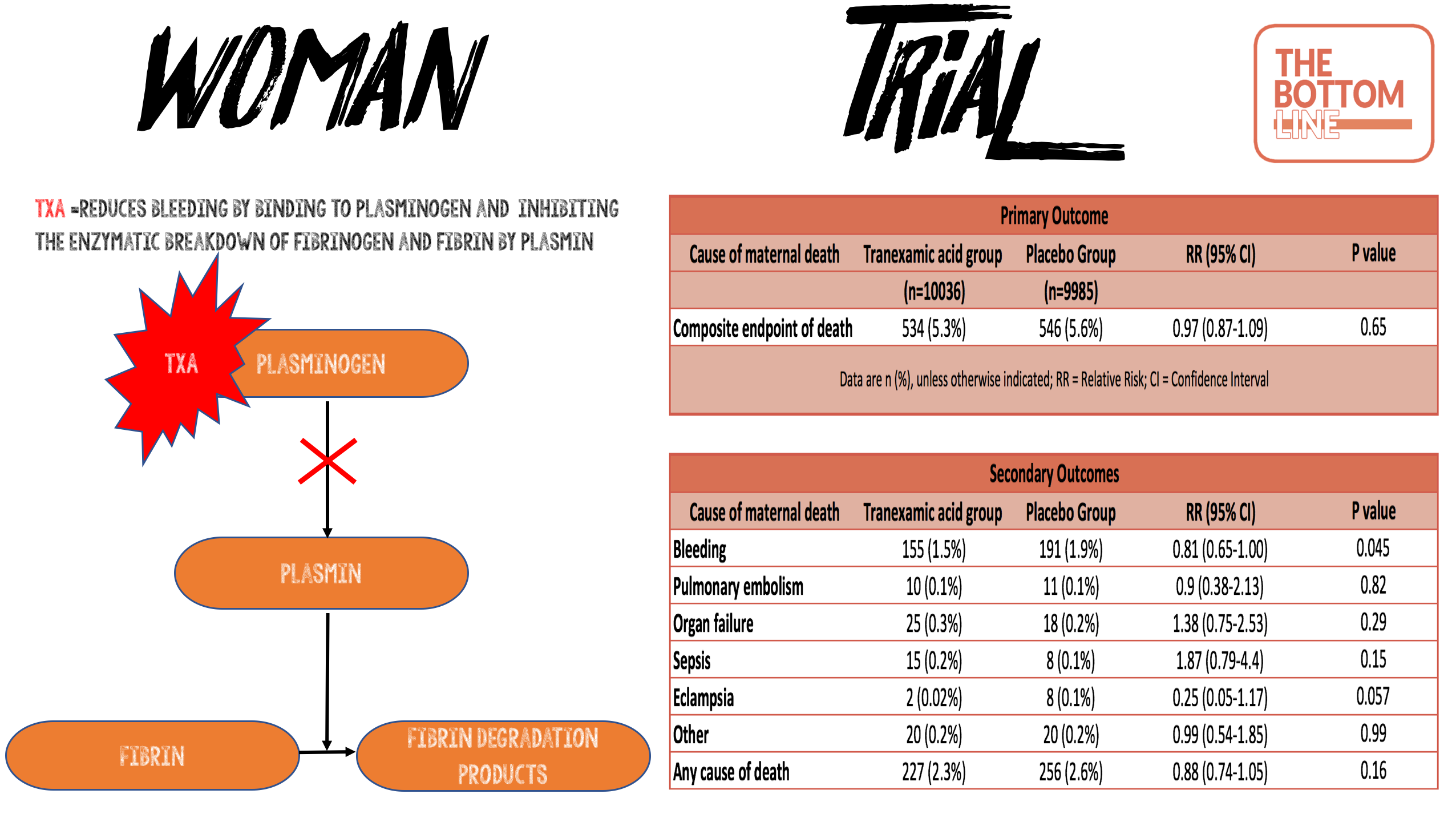
- TXA acid reduces bleeding by inhibiting the enzymatic breakdown of fibrinogen and fibrin by plasmin
- CRASH-2 demonstrated that TXA reduces all cause death at 4 weeks in trauma patients (Absolute Risk Reduction 1.5%). A subgroup analysis demonstrated a 0.8% ARR reduction in death secondary to bleeding with TXA
Design
- Randomised Controlled Trial
- Double-blinded
- Sealed envelopes containing randomisation codes
- Intention to treat analysis
- A sample size of 20,000 patients was calculated to provide sufficient power to detect an absolute reduction in mortality from 3% to 2.25% (25% relative risk reduction) at the 5% significance level
Setting
- 193 hospitals in 23 countries
- March 2010 – April 2016
Population
- Inclusion: women aged 16 years or over with a clinical diagnosis of PPH after a vaginal birth or caesarian section
- Exclusion: clinician decision that it would be inappropriate for individual to receive or not receive tranexamic acid or some other treatment could be offered outside the trial
- 20060 enrolled
- Baseline characteristics were very well balanced
- Included age, type of delivery (71% vaginal; 29% caesarean section); placenta fully delivered in 90% cases; primary cause for haemorrhage (uterine atony 2/3rds), and haemodynamic parameters
- Estimated blood loss was between 500-1000ml in 50% of cases. Estimated blood loss was <1500ml in 80% of cases
Intervention
- Tranexamic Acid (n=10036)
- 1g (10mg/ml) administered intravenously over 10 minutes
Control
- Placebo (n=9885)
- Identical packaging and volume administered intravenously over 10 minutes
In both groups
- If bleeding continued after 30 minutes or stopped and then re-started within 24 hours of the first dose, a second dose of study drug was administered
Outcome
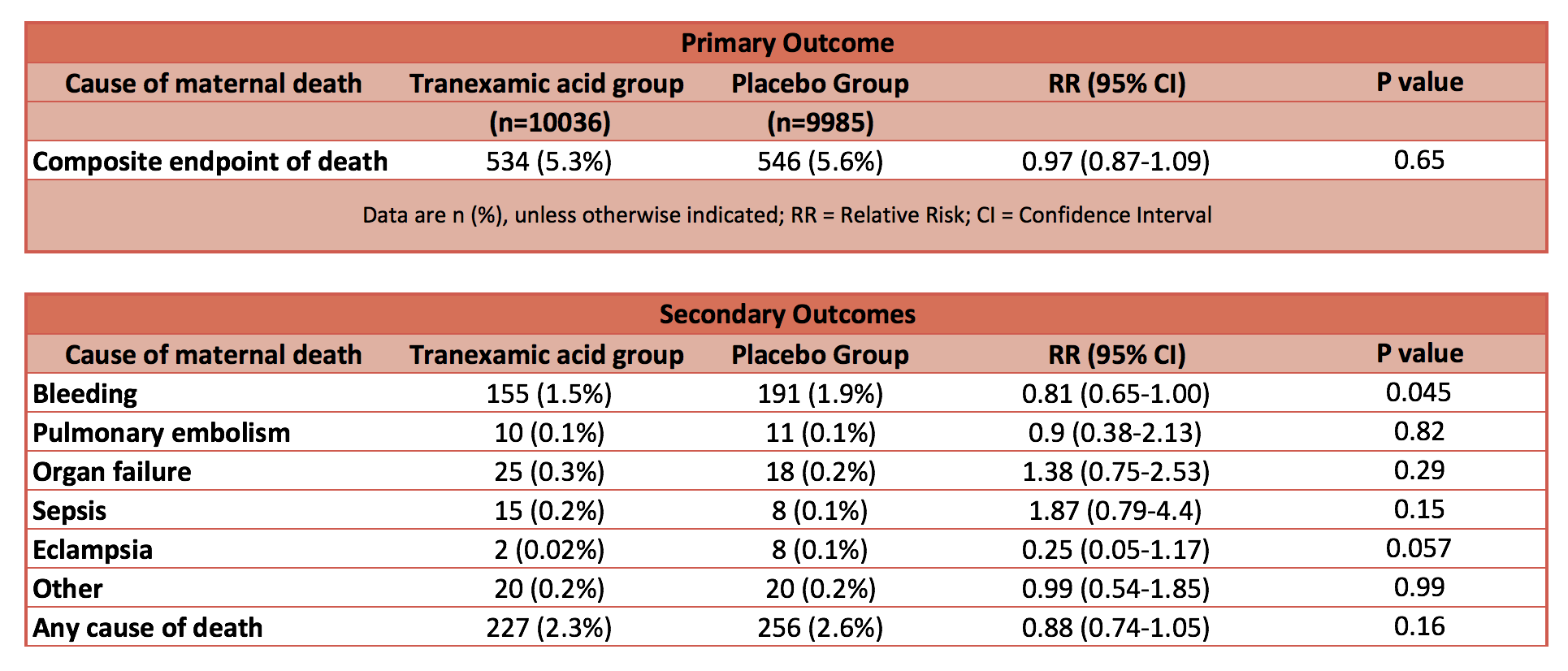
- Primary outcome: Composite endpoint of death from all causes or hysterectomy within 42 days of randomisation: No statistical difference
- 5·3% in TXA group vs 5·5% in the placebo group
- 95% CI 0·87-1·09; p=0·65
- Secondary outcomes:
- Death due to bleeding: statistically significantly reduced in TXA group
- 155 (1·5%) of 10 036 vs 191 (1·9%); risk ratio [RR] 0·81, 95% CI 0·65–1·00; p=0·045
- Absolute risk reduction 0.4%; NNT 267; Fragility Index 0
- After adjusting for baseline risk, the RR for death due to bleeding with TXA was 0·78 (95% CI 0·62–0·98; p=0·03)
- Other sub-group causes of death:
- ‘Any cause’ of death: reduced mortality 2.3% vs 2.6% in the TXA group but no statistical difference P=0.16
- Pulmonary embolism; organ failure; sepsis; eclampsia: No statistical difference
- Other secondary outcomes: no statistical difference in:
- Thromboembolic events (deep-vein thrombosis, pulmonary embolism, myocardial infarction, and stroke)
- Surgical interventions (intrauterine tamponade, embolisation, brace sutures, arterial ligation, hysterectomy, and laparotomies done after randomisation to control bleeding and achieve haemostasis)
- Complications (renal failure, cardiac failure, respiratory failure, hepatic failure, sepsis, and seizures), other untoward medical events (adverse events)
- Quality of life measured using the EQ5D
- Death due to bleeding: statistically significantly reduced in TXA group
- Timing of tranexamic acid
- TXA within 3 h of giving birth
- reduced death 1·2% vs 1·7% RR 0·69, 95% CI 0·52–0·91; p=0·008
- TXA after 3 h of giving birth
- increased death 2·6% vs 2·5% RR 1·07, 95% CI 0·76–1·51; p=0·70
- No heterogeneity in the effect by type of birth or cause of bleeding
- TXA within 3 h of giving birth
Effects of TXA on maternal death
Authors’ Conclusions
- Tranexamic acid reduces death due to bleeding in women with post-partum haemorrhage with no adverse effects. When used as a treatment for post-partum haemorrhage, tranexamic acid should be given as soon as possible after bleeding onset
Strengths
- Large, multicentre, international RCT
- Ampoules and packaging were identical in appearance and prepared by an independent pharmaceutical company
- Correct masking and coding of ampoules was checked by independent random testing of each batch by high-performance liquid chromatography to confirm the contents of the ampoules
- Only 33 patients (0.16%) were lost to follow-up
- Trial is registered with ClinicalTrials.gov (NCT00872469)
Weaknesses
- Two important design changes occurred with the study after it was commenced
- Sample size increased by 5000 patients during the study. The rationale for this was based on an anticipated baseline event rate of 2.5% for death and 2.5% for hysterectomy and that 1% of women die after hysterectomy. However, during the trial it became apparent that the decision to conduct a hysterectomy was often made at the same time as randomisation. Although TXA could influence the risk of death in these cases, it could not affect the risk of hysterectomy. The sample size was therefore increased from 15,000 to 20,000 with the intention that this might compensate for the dilution of the treatment effect from hysterectomies that were done at the same time as randomisation and that the study had enough power to detect a reduction in death from PPH
- Study hypothesis was refined after commencing the study in light of outcome findings from the CRASH-2 study (published in 2011). The authors explain that mortality with death due to bleeding was changed to the main secondary outcome. This was done before un-blinding and without knowledge of the trial results
- There was no formal screening for embolic complications and perhaps these were missed, particularly as follow-up was limited to hospital discharge or on 42 days if still in hospital
The Bottom Line
- Tranexamic acid may be beneficial in reducing the risk of death due to post-partum haemorrhage. The study has some significant methodological limitations, including a change in power calculation and hypothesis after it was commenced, as well as a low fragility index. On the basis of it being cheap with a good safety profile in this study, consider giving tranexamic early in post-partum haemorrhage. Early resuscitation, management of coagulopathy and surgical assistance with source control, are the most important interventions
Infographic
External Links
- [Paper] WOMAN trial – Lancet
- [Editorial] WOMAN: reducing maternal deaths with tranexamic acid
- [videocast] WOMAN – TXA History
- [further reading] BroomeDocs – Thoughts on the WOMAN Trial
- [further reading] CRASH-2
Metadata
Summary author: Steve Mathieu
Summary date: 2nd May 2017
Peer-review editor: Celia Bradford




One significant confounder to applying these findings to my practice in the UK is that only 569 patients were in the UK. The mortality from haemorrhage in the UK is significantly below 1.5-1.9% so it would be interesting to see that sub-group analysis of UK patients.