Anderson

Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke
Anderson CS et al. NEJM 2016 DOI: 10.1056/NEJMoa1515510
Clinical Question
- In patients with acute ischaemic stroke, is low-dose thrombolysis therapy with intravenous alteplase non-inferior to standard-dose therapy with respect to death and disability at 90 days?
Design
- One aspect of Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED) which also looks at early intensive lowering of BP
- Prospective, randomized
- Open-label trial with blinded outcome assessors
- Non-inferiority
- Follow up evaluation carried out in person or by telephone by trained and certified staff
- Sample size 3300 patients: 90% power for detecting non-inferiority relative margin of 14%, assuming 5% dropout
Setting
- International, multicentre
- 111 centres in 13 countries; Asia, Australia/New Zealand, Europe and South America
- March 2012 – August 2015
Population
- Inclusion:
- ≥18 years with a clinical diagnosis of acute ischemic stroke confirmed by brain imaging
- able to receive thrombolysis treatment within 4.5 hours of symptom onset
- were previously independent, as defined by a prestroke functional ability of 1 or less on the modified Rankin scale (mRS)
- systolic blood pressure ≤185 mmHg
- patients with higher BP can still be included provided the BP is reduced to the entry level prior to commencement of alteplase
- Exclusion
- unlikely to benefit from the therapy due to pre-existing disability (e.g. advanced dementia) or very high likelihood of death within 24 hours
- another medical illness that interferes with outcome assessments
- unlikely to adhere to follow-up procedures
- no consent to participate
- previously enrolled in ENCHANTED
- 69305 screened, 3310 randomised
Intervention:
- Low-dose group (n=1650)
- rtPA 0.6 mg/kg i.v. alteplase, maximum total dose of 60mg
- 15% bolus (maximum bolus dose of 9mg)
- infusion of the remainder of the total dose over 60 minutes
Control:
- Standard-dose group (n=1643)
- rtPA 0.9 mg/kg i.v. alteplase, maximum total dose of 90mg
- 10% bolus (maximum bolus dose of 9mg)
- infusion of the remainder of the total dose over 60 minutes
Outcome
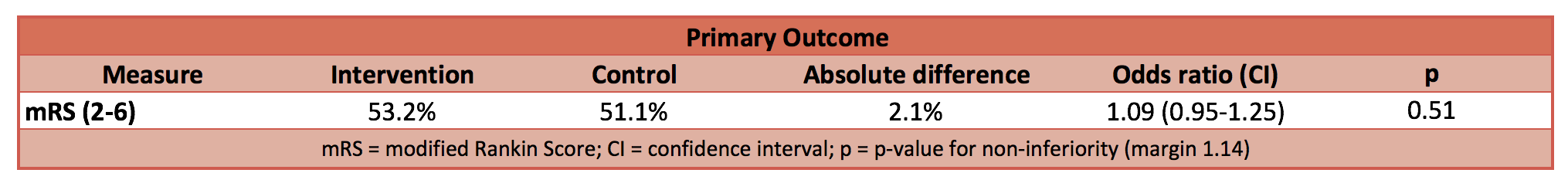
- Primary: No significant difference in modified Rankin Score of 2-6 (indicates a poor outcome with increasing degree of disability and death)
- Secondary:
- Incidence of symptomatic intracranial haemorrhage reduced in the low-dose alteplase group
- Mortality at 7 days reduced in low dose group: 3.6% vs 5.3% (odds ratio, 0.67; 95% CI, 0.48 to 0.94; P=0.02)
- No significant difference between groups in incidence of:
- Death of major disability
- Death within 90 days
- Overall health utility score on EQ-5D
- Admission to residential care
- Median duration of hospitalization
- Death or neurological deterioration within 72 hours
- Serious adverse events
Authors conclusion
- In patients with acute ischaemic stroke who were eligible for thrombolysis, low-dose alteplase was not shown to be non-inferior to standard dose in terms of death and disability
Strengths
- International, multicentre
- Assessor blinded
- Thrombolysis protocol based on most commonly adopted practice
Weakness
- Over 60% of patients recruited were Asian which may limit external validity
- Not powered to detect difference between Asian and non-Asian population
- Main reasons patients with ischaemic stroke were excluded was inability to deliver thrombolysis therapy within 4.5 hours
- Approximately 10% of patients in both groups were excluded in final per-protocol analysis
- No consideration of occlusion site or interventional recanalisation strategies
The Bottom Line
- Data from previous meta-analysis suggest an increase risk of symptomatic and fatal intracranial haemorrhage with the use of thrombolysis. This trial fails to demonstrate non-inferiority (or superiority) of low-dose alteplase compared to standard-dose. With the results of this trial, standard dose alteplase should remain in institutions that are able to provide thrombolytic therapy for acute ischaemic stoke.
External links
- [Article] Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke
- [Further reading] Supplementary material
- [Further reading] Thrombolysis in acute ischaemic stroke: time for a rethink?
- [Further reading] LITFL: Stroke Thrombolysis
- [Further reading] R.E.B.E.L EM: The ENCHANTED Trial: Is Low-Dose the Right Dose for Intravenous tPA in Acute Ischemic Stroke?
- [Further reading] EMNerd (EMCrit)The Case of the Non-inferior Inferiority Continues
Metadata
Summary author: Adrian Wong
Summary date: 25th May 2016
Peer-review editor: Dave Slessor




Pingback: The ENCHANTED Trial: Is Low-Dose the Right Dose for Intravenous tPA in Acute Ischemic Stroke? - R.E.B.E.L. EM - Emergency Medicine Blog
I’m confused, no change in primary outcome, reduction of harm clear in secondary outcomes. How is it not superior?
Howard, thanks for your comment. Your point is not an unreasonable one, and that is one way to interpret these results for your patients. I’ll explain my view in simple terms for any reader, but I apologise if this is obvious to you.
I’ve been through the primary outcome data and re-calculated the risk ratio and odds ratio with 95% CI to double check their outcome. The RR is 1.04 (95% CI 1.11–0.97) and OR is 1.09 (95% CI 1.25–0.95). Which statistical description you choose is up to you – the authors of this trial chose to describe it in terms of odds. They defined a threshold that, if breached by the 95% CI limits, non-inferiority could not be concluded. The upper limit for the odds ratio is 1.25, which is beyond the 1.14 limit set by the authors. And since the 95% CI include 1.00, superiority or inferiority cannot be concluded either.
Regarding secondary outcomes, there is superiority demonstrated with regards to death at 7 days and reduced intracranial haemorrhage, but since this isn’t seen at 90 days, I am cautious about accepting it as a true positive outcome in favour of a reduced thrombolysis dose.
In summary, the biostatistical conclusion has to be that neither superiority nor non-inferiority has been demonstrated. How you want to interpret the detail is up to you!
Please reply if you have any further thoughts on this.
– Duncan (Editor for TBL)