DanGer Shock – Microaxial Flow Pump in Infarct-Related Cardiogenic Shock

Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock
Møller JE et al. 2024. NEJM. DOI: 10.1056/NEJMoa2312572
Clinical Question
- In adults presenting with STEMI and cardiogenic shock does the use of a microaxial flow pump (Impella CP) compared to standard care reduce death from any cause at day 180?
Background
- Cardiogenic shock is a frequent complication of STEMI and is associated with significant morbidity and mortality
- Microaxial flow pumps (MAFP) are percutaneously inserted devices that pump blood out from the left ventricle into the ascending aorta
- Prior RCTs have been small and not shown any benefit with a signal towards some harm (bleeding) in registry studies
Design
- International, multi-center RCT
- Open label
- Randomised via web-based system and stratified by localization of STEMI and timing relative to revascularisation
- Randomisation occurred in the catheter laboratory either before or after revascularisation (could occur up to 12 hours after leaving) depending on when cardiogenic shock was recognised
- Sample size calculation
- 360 patients required to detect a reduction from 60% to 42% in 180 day mortality with 80% power at an alpha of 0.05
- Pre-defined subgroups
- Registered with clinicaltrials.gov
Setting
- 14 centers in Denmark (4 centers), Germany (9) and UK (1)
- January 2013 to July 2023
Population
- Inclusion:
- ≥ 18 years old
- STEMI
- STEMI equivalent also allowed
- Cardiogenic Shock
- SBP < 100mmHg +/- need for ongoing vasopressor support AND lactate > 2.5 mmol AND LV Ejection Fraction < 45%
- Exclusion:
- Resuscitated from out-of-hospital cardiac arrest who remained comatose (GCS < 8)
- Severe right ventricular failure (RV larger than LV, TAPSE < 1cm or septal shift)
- Shock duration > 24 hours
- Other causes of shock or shock due to mechanical complication of MI
- Severe aortic regurgitation or stenosis
- Severe arterial disease precluding placement
- 1211 screened → 360 enrolled
- 851 excluded or which only 39 were eligible and not randomised
- 5 excluded following randomisation as consent unable to be obtained
- Comparing baseline characteristics of MAFP vs. standard care group
- Well balanced
- Age: 67 vs 69
- Male: 79 vs 79%
- SBP: 84 vs 82 mmHg
- Lactate: 4.6 vs 4.5 mmol/L
- LVEF: 25 vs 25%
- Resuscitation prior to randomisation: 22 vs 19%
- Anterior MI: 70 vs 73%
- SCAI
- C: 56 vs 55%
- D: 29 vs 28%
- E: 16 vs 17%
- Number of disease vessels on angiography:
- 1: 29 vs 27%
- 2: 39 vs 36%
- 3: 31 vs 37%
- Time from symptom onset to randomisation: 4.8 vs 3.8 hours
- Randomised prior to revascularisation: 55 vs 58%
- In-hospital management of cardiogenic shock largely similar:
- PCI: 96 vs 98%
- Time from admission to balloon inflation: 58 vs 45 mins
- Placement of Impella CP: 95 vs 2%
- Median duration of mechanical ventilation: 5 vs 3 days
- Vasoactive use:
- Noradrenaline: 87 vs 81%
- Dopamine: 29 vs 23%
- Adrenaline: 37 vs 38%
- Dobutamine: 35 vs 34%
- Milrinone: 35 vs 33%
- Levosimendan: 22 vs 22%
- Median duration of ICU admission: 6 vs 3 days
- Median duration of hospital admission: 12 vs 7 days
Intervention
- Insertion of MAFP
- Inserted immediately after randomisation
- Run at the highest performance level for 48 hours unless complications occurred
- Criteria and guidance for weaning provided
- Median duration of MAFP support: 59 hours
- 8 did not receive a MAFP
Control
- Standard Care – no use of a MAFP
- 3 crossed over to MAFP
Management common to both groups
- All participants underwent a revascularisation procedure as needed
- In event of haemodynamic instability treatment could be escalated
- In microaxial group this was Impella 5.0 or Impella RP or ECMO
- In control group ECMO was recommended but Impella 5.0 allowed
- If an Impella was used in this manner this was defined as a protocol violation
- This occurred in 16% in microaxial pump group and 21% in standard care group (largely to VA ECMO)
Outcome
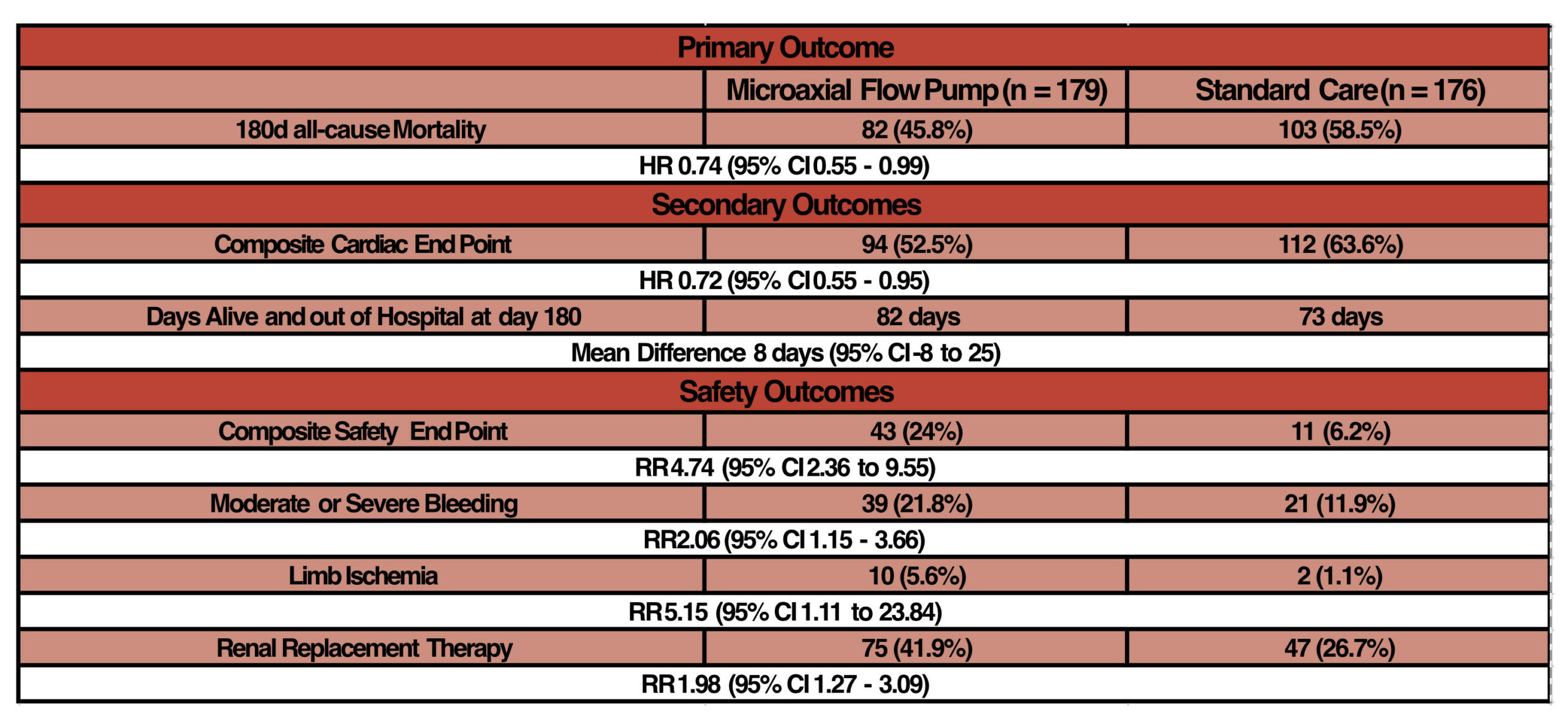
- Primary outcome: Death from any cause at 180 days
- 45.8 (microaxial flow pump) vs 58.5% (standard care)
- HR 0.74 (95% CI 0.55 to 0.99)
- NNT = 8
- 45.8 (microaxial flow pump) vs 58.5% (standard care)
- Secondary outcomes:
- Composite cardiac end point (escalation to additional mechanical support, heart transplantation or death)
- 53 vs 64%; HR 0.72 (95% CI 0.55 to 0.95)
- Number of days alive and out of hospital at day 180:
- 82 vs 73; Mean difference 8 (95% CI -8 to 25)
- Composite cardiac end point (escalation to additional mechanical support, heart transplantation or death)
- Safety Outcomes:
- Composite safety end point (severe bleeding, limb ischaemia, haemolysis, worsening aortic regurgitation, device failure)
- 24 vs 6%; RR 4.74 (95% CI 2.36 – 9.55)
- RRT
- 42 vs 27%; RR 1.98 (95% CI 1.27 – 3.09)
- Composite safety end point (severe bleeding, limb ischaemia, haemolysis, worsening aortic regurgitation, device failure)
- Subgroups
- The following had 95% CI in favour of micro-axial flow pump
- Male: HR 0.66 (95% CI 0.47 – 0.93)
- Mean Arterial Pressure < 64 mmHg: HR 0.61 (95% CI 0.41 – 0.92)
- ≥2 disease vessels: HR 0.68 (95% CI 0.49 – 0.94)
- The following had 95% CI in favour of micro-axial flow pump
Authors’ Conclusions
- Routine use of a microaxial flow pump in the treatment of patients with STEMI related cardiogenic shock led to a lower risk of death at 180 days. The incidence of adverse events was higher
Strengths
- Excellently conducted RCT with high internal validity
- Rapid placement of device following randomisation (14 minutes) with short door to balloon times
- A seemingly sick cohort with a median SBP in the 80s and lactate of 4.5, with well-balanced baseline characteristics
- Similar use of other ICU supports between groups
- Minimal protocol violations
- Minimal loss to follow up
- Clear guidelines on weaning of Impella support
- Excellent data on safety outcomes with clear definitions of what constitutes an adverse event
Weaknesses
- Unblinded
- When as-treated populations analysed confidence intervals lose significance (HR 0.77, 95% CI 0.57 – 1.03) (Figure S4)
- Single centre in UK may limit external validity
- Figure S2 shows the heterogeneity of treatment effect across enrollment countries (Denmark vs Germany and UK) favours Denmark (not adjusted for multiplicity)
- It is important to understand how long these devices have been used in each country to understand how and if volume of practice affects outcomes
- Slow enrollment – 10 years to complete recruitment
- No obvious difference in subgroup analysis
- Data on safety outcomes doesn’t account for competing risk (e.g. if you die you can’t have RRT)
- Does running on the highest support for 48 hours affect the risk of haemolysis?
- This might increase AKI and need for RRT as hypothesised by the authors
- It might be that the patients needed less support (lower setting) than the maximal settings to achieve adequate organ support, which may result in lower rates of haemolysis
- However, within the settings of a clinical trial it is understandable to protocolise these settings
- May be hard to directly compare with other trials looking at mechanical cardiac supports (e.g. ECLS Shock)
- This trial didn’t include those comatose post cardiac arrest
- Given this population will have a higher rate of neurological injury and therefore withdrawal of life sustaining therapies mortality rates may be different
- Low rates of neurological causes of death (6 patients total, Table S3) were demonstrated in this trial
- This trial didn’t include those comatose post cardiac arrest
- Guidelines for other ICU support provided in protocol but no data provided on adherence (e.g. anticoagulation)
- Some industry involvement and support (Abiomed)
The Bottom Line
- It is hard to ignore this trial. Whilst acknowledging the increased rates of adverse events, the mortality benefit is striking
- This trial should promote multi-disciplinary discussions around the potential benefit for MAFP use within local healthcare settings
External Links
- article Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock
- editorial Mechanical Circulatory Support in Cardiogenic Shock — Persistence and Progress
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 12th April 2024
Peer-review editor: @davidslessor
Picture by: AI Generation Microsoft Co-Pilot



