HYPRESS
Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis
Keh. JAMA 2016. Published online October 3, 2016.doi:10.1001/jama.2016.14799
Clinical Question
- In patients with severe sepsis does hydrocortisone compared to placebo prevent the development of septic shock?
Design
- Randomised, double-blind, placebo-controlled, multicentre trial
- Internet-based randomisation stratified by participating centre and sex
- The randomization used the Pocock minimization algorithm to ensure balanced 1:1 randomization in the strata at any time
- All patients, study personnel, staff were blinded for the entire study
- Intention to treat (and per protocol) analysis
- Assuming 40% of the patients in the placebo group would develop septic shock, to detect a 15% difference with the intervention arm (p<0.05, power of 80%), 169 patients per arm were required. Accounting for a drop out of ~10%, 190 patients per arm (380 total) were included.
Setting
- 34 study sites in Germany
- January 13 2009 to August 27 2013
Population
- Inclusion:
- Evidence of infection (1 of: micro-organism identified in normally sterile body fluid, identified focus of infection, granulocytes in sterile body fluid, clinically suspected infection without microbiological evidence)
- Evidence of SIRS (2 of: fever >38°C or hypothermia <36°C, tachycardia >90bpm, tachypnea >20/min or CO2<33mmHg or mechanically ventilated, leukocytosis >12000/ul or leucopenia <4000/ul or >10% immature forms)
- Evidence of Organ Dysfunction for not longer than 48 hours (1 of: encephalopathy, AKI, coagulopathy, pulmonary dysfunction, microcirculatory dysfunction)
- Informed consent possible from patient or NOK
- Exclusion:
- Patients with septic shock, ie patients who are hypotensive despite adequate fluid resuscitation (MAP<65Hg, SBP<90mmHg) or those needing vasopressors for more than 4 hours. Transient use of vasopressors ok whilst fluid resuscitation occurs.
- Patients with hypersensitivity to the hydrocortisone or placebo (mannitol)
- Patients regularly on glucocorticoids
- Patients with a condition indicating glucocorticoid therapy
- DNR or moribund patients
- <18 years
- Recent trial participation (30 days)
- Pregnant/Breast-feeding
- Related to study personnel
- Patients were NOT EXCLUDED for using etomidate or a short course of glucocorticoids within 72 hours before enrollment OR using topical or inhaled glucocorticoids
- 9953 patients with severe sepsis or septic shock were screened and 380 randomized to receive hydrocortisone n=190 or placebo n=190
Intervention
- Bolus of hydrocortisone iv 50mg followed by a 24 hour continuous infusion of 200mg for 5 days, 100mg for Day 6 & 7, 50mg on Day 8 & 9 and 25mg on Day 10 & 11
- The continuous infusion was preferred to prevent unwanted undulation in blood glucose concentrations
Control
- The placebo was lyophilized mannitol which was indistinguishable from the hydrocortisone (133mg mannitol – a tiny dose compared with therapeutic mannitol for raised ICP = 1g/kg)
Outcome
- Primary outcome: the occurrence of septic shock within 14 days, which was assessed daily until day 14 or discharge from ICU
- The intention to treat analysis excluded 27 patients – consent issues, septic shock at inclusion, or did not receive study medication
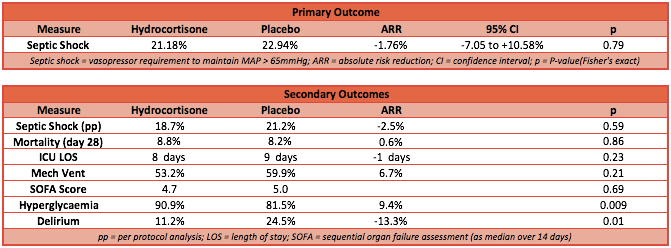
- In the ITT population: shock occurred in 36/170 (21.2%) patients in the hydrocortisone group vs 39/170 (22.9%) patients in the placebo group (p=0.70, Difference= -1.8% 95% CI -10.7% to 7.2%)
- In the per-protocol analysis there was no difference in development of septic shock
- Subgroup analysis: medical vs surgical patients, pneumonia, those receiving study medication for> 48hrs did not reveal any benefit for shock prevention
- Secondary outcome: No differences between groups:
- Time until development of septic shock or death
- Mortality in ICU and hospital
- Vital status at Day 28, 90 & 180
- Duration of ICU and hospital stay
- SOFA score
- Duration of mechanical ventilation
- RRT
- In 206 patients, baseline cortisol concentration was checked and the level rechecked following administration of 250ug corticotropin. The primary and secondary outcomes in this subgroup were evaluated – 33.5% of these patients had CIRCI (Critical Illness Related Corticosteroid Insufficiency). No difference in primary or secondary endpoints between patients with or without CIRCI
- Adverse events assessed included muscle strength scores, secondary infection, hyperglycaemia, gastrointestinal bleeding, delirium and weaning failure. There were more episodes of hyperglycaemia in the hydrocortisone group but the total amount of insulin delivered was not significantly different. Delirium was less common in the hydrocortisone group (placebo group 24.5% vs hydrocortisone group 11.2%, p=0.01). The other adverse events did not differ between groups.
Authors’ Conclusions
- Administration of hydrocortisone did not prevent the development of shock in patients with severe sepsis.
Strengths
- An important question: the use of steroids in sepsis and septic shock is one of the longest running debates in critical care. This trial uniquely examines the use of steroids to prevent shock in patients with established sepsis.
- Allocation concealment
- Blinding
- Intention to treat analysis
- <5% lost to follow-up
Weaknesses
- Patients who developed septic shock early may have been missed because informed consent was necessary before randomisation
- The mortality rate in this trial was relatively low (8.5% at Day 28) so this was a relatively well cohort compared to other sepsis trials (reflecting the haemodynamic stability of the patients at the time of randomization)
- Not all patients had baseline adrenal function assessed. Only certain sites did this test and it needed to be done before randomization occurred.
- Etomidate was used in 6.3% (placebo group) and 6.8% (hydrocortisone group) of patients pre-randomization. Etomidate selectively inhibits adrenal corticosteroid synthesis which may impact the overall result
- Patients in the placebo arm were more likely to have received glucocorticoids pre-randomization and at higher doses (3.4% vs 1.7%, 600mg vs 200mg), but the number of patients involved was small.
The Bottom Line
- As a non-believer in the use of steroids in sepsis and septic shock I will continue my current practice of not using steroids in this setting.
- I am working at a hospital actively recruiting for the ADRENAL trial. 3800 patients with septic shock are being recruited in a placebo-controlled trial to assess 90 day mortality. I keenly await these results which may put this question to rest forever!
External Links
- [article] HYPRESS RCT
- [further reading] ADRENAL trial protocol
- [further reading] Podcast: Cohen Steroids in Sepsis
Metadata
Summary author: Celia Bradford
Summary date: October 12 2016
Peer-review editor: Duncan Chambler




Pingback: The HYPRESS Trial: Early Steroids to Prevent Septic Shock - R.E.B.E.L. EM - Emergency Medicine Blog
Pingback: SGEM#168: HYPRESS – Doesn’t Got the Power | The Skeptics Guide to Emergency Medicine
Do you have any idea why the trial used mannitol as it’s placebo? Isn’t mannitol an osmotic diuretic that would hypothetically lower blood pressure further??
Jenny,
Thank you for your comment. It’s a good question. I’m guessing that lyophilised mannitol has a similar consistency and colour to the drug, to make it indistinguishable. The dosage, as Celia noted, is tiny compared to a therapeutic mannitol dose (less than 1%), so I don’t think it can lead to lower blood pressure. Trials are so carefully considered when being designed, so I doubt the authors would have done this if they weren’t sure. That’s not a particularly critical or scientific answer from me though!