TTM2

Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest
Dankiewicz J for the TTM2 Trial Investigators. NEJM June 17, 2021 DOI:10.1056/NEJMoa2100591
Clinical Question
- In patients who are unconscious following out-of-hospital cardiac arrest, does targeted hypothermia compared with targeted normothermia, impact all-cause mortality?
Background
- There have been several randomised trials investigating temperature management following cardiac arrest
- The HACA trial (n=137) and Bernard trials (n=77) published in NEJM in 2002 reported that therapeutic hypothermia following a VF arrest improved favourable neurological outcome. However, small sample sizes and other methodological flaws meant the evidence was of low certainty. A number of patients in the control group developed fever and it was therefore unclear if the reported benefits were from hypothermia or the avoidance of fever
- The TTM (2013) study (n=950) compared a targeted temperature of 33°C vs. 36°C in patients with an out-of-hospital cardiac arrest from a presumed cardiac cause. They reported no significant difference in all-cause mortality
- The HYPERION trial (n=584) showed that in patients with coma following a non-shockable cardiac arrest, the use of moderate hypothermia improved favourable neurological outcome at 90 days compared with targeted normothermia
- The European Resuscitation Council released new guidelines in March 2021. These suggest:
- maintaining a target temperature at a constant value between 32 and 36°C for at least 24 h
- avoidance of fever (> 37.7°C) for at least 72 h after ROSC in patients who remain in coma
- Fever is associated with worse outcomes
Design
- International, multicentre, parallel group trial
- Randomised, superiority trial
- Co-enrolment with the TAME trial (Targeted Therapeutic Mild Hypercapnia)
- Randomisation occurred in the emergency department
- Web-based allocation using permuted blocks of varying sizes (stratified by site + TAME randomisation) in 1:1 ratio
- Pre-specified secondary outcomes (neurological outcome and health related quality of life) and subgroup analyses (sex, age, time to ROSC, initial rhythm and whether shock present on admission)
- Outcomes assessed at 30 days, 6 months, 24 months (ongoing)
- Blinding:
- Non blinded clinicians, however assessors of prognosis, participants, outcome assessors, statisticians, data managers blinded.
- Duplicate manuscripts were written for each scenario before randomisation was revealed
- Outcome assessment was by face-to-face interview (72%) or telephone interview (particularly used when the COVID-19 pandemic began). Where possible the patient and a proxy were used to obtain information
- MRS was performed via a structured interview with interviewers receiving formal training
- Power Calculation: A total of 1862 participants gave a power of 90% (alpha 0.05, NNT 13.3) to detect an absolute risk reduction of 7.5% in mortality (assuming 55% mortality). This was increased to 1900 to account for loss to follow-up
Setting
- Number of countries: 14 countries, 61 institutions
- Highest recruiting countries: Sweden, UK, Switzerland, France, Czech Republic
- Dates of randomisation: pilot phase began late 2017. Active recruiting through 2018 to early 2020
- Last data collection: 6-month follow-up completed mid 2020 with 24 month follow-up ongoing
Population
- Inclusion:
- Out-of-hospital cardiac arrest of presumed cardiac or unknown cause
- Sustained ROSC: >20 minutes of circulation without need for chest compressions
- Unconscious: as defined by the FOUR score motor response <4 and not able to obey verbal command
- No limitations to management in place
- Inclusion must be no later than 180 minutes after ROSC
- Exclusion:
- Unwitnessed cardiac arrest with initial rhythm asystole
- Temperature on admission < 30°C
- On ECMO prior to ROSC
- Suspected pregnancy
- Intracranial bleed
- Severe COPD with long-term home O2
- 4355 patients screened.
- 55% not randomised due to exclusion criteria:
- most commonly >180 minutes since ROSC, non-cardiac cause for arrest, not unconscious, limitations to care in place
- 1900 randomised
- 1861 analysed as intention to treat (39 withdrew consent)
- 930 in Hypothermia arm, 931 in Normothermia arm
- 1850 analysed for survival (11 lost to follow-up)
- 55% not randomised due to exclusion criteria:
- Baseline characteristics between groups (Hypothermia vs Normothermia). No significant difference in:
- Age (mean 64 vs. 64)
- Sex (80% male vs. 79%)
- Shockable Rhythm (72% vs. 75%)
- Bystander CPR (82% vs. 78%)
- Shocked on admission (28% vs. 30%)
- Defined as SBP < 90mmHg for > 30 minutes or evidence of end-organ hypoperfusion
- Median time to ROSC (25 minutes vs. 25 minutes)
- Temperature on admission (35.3°C vs. 35.4°C)
- Lactate (5.9 vs 5.8)
- STEMI (41% vs 40%)
Intervention
- Hypothermia group: target temperature 33C
- Rapid cooling achieved by cold fluids and physical cooling devices (surface or intravascular devices).
- A feedback-controlled system used to maintain target temperature (bladder temperature probe)
- Rewarming began after 28 hours (increase temperature by no more than 0.3°C per hour until 40 hours)
- 95% of patients received cooling
- 70% cooled with external devices and 30% with intravascular devices
Control
- Normothermia group: In participants who developed a temperature of 37.8°C (trigger), a device was used and set at 37.5°C
- Pharmacological and conservative therapies initiated first
- Physical cooling devices (surface or intravascular) used if temperature ≥ 37.8°C
- Physical cooling devices could be placed prophylactically, but no active cooling was provided for patients who had a spontaneous temperature of <37.8C
- A feedback-controlled system used to maintain target temperature
- 46% of patients needed a device to achieve target temperature (of these 69% used surface cooling, and 31% used intravascular cooling)
- In 1st 24 hours ~5% of temperatures recorded at >38C
Management common to both groups
- Normothermia from 40 – 72 hours (36.5°C – 37.7°C) – temperature management could be initiated after 40 hours
- Sedation mandated in both groups until the end of the intervention period (40 hours)
- Protocolised step-wise protocol for treatment of shivering
- At 96 hours a neurological assessment was performed by a blinded clinician – only following this was withdrawal of life sustaining treatments allowed for presumed poor neurological outcome.
- Standardised criteria for what was considered a likely poor neurological outcome were used
- Other than above patients were managed as per clinician preferences. There were no differences (hypothermia vs normothermia) in:
- Cardiac catheter rates: 78% vs. 78%
- 90% vs 87% within 2 hours
- PCI: 38% vs. 38%
- CAGs: 1% vs 2%
- AICD: 16% vs 16%
- Cardiac catheter rates: 78% vs. 78%
Outcome
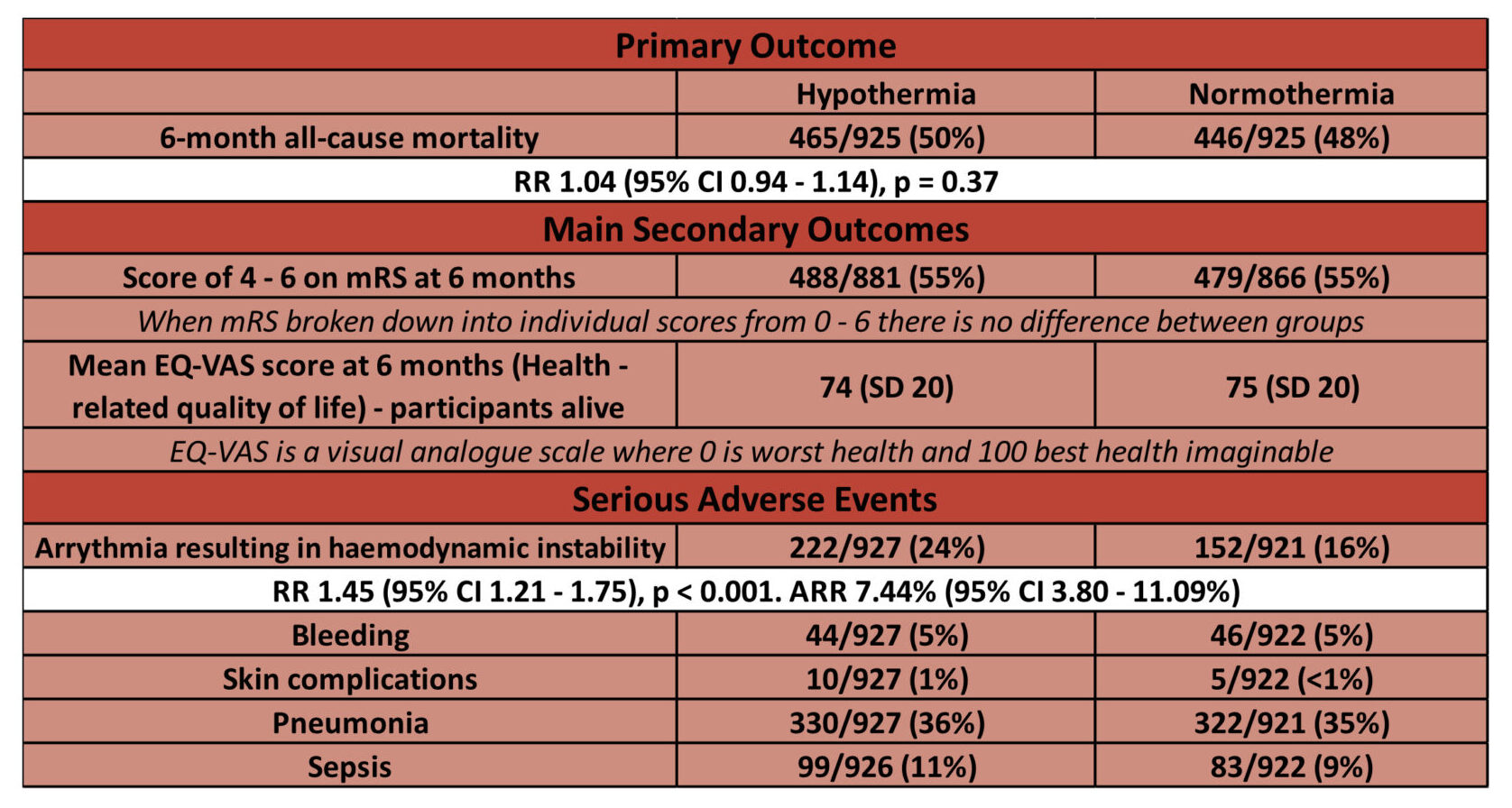
- Primary outcome: No difference in all-cause mortality at 6 months
- Comparing HYPOTHERMIA vs NORMOTHERMIA
- 50% (465/925) vs 48% (446/925)
- RR 1.04 (95% CI 0.94-1.14, p = 0.37)
- HR for death in hypothermia group 1.08 (95% CI 0.95-1.23)
- 50% (465/925) vs 48% (446/925)
- Comparing HYPOTHERMIA vs NORMOTHERMIA
- Secondary outcomes: No difference in secondary outcomes
- Comparing HYPOTHERMIA vs. NORMOTHERMIA
- Proportion of people with a poor functional outcome (modified Rankin scale, mRS 4-6) at 6 months: 55% vs 55% (RR 1.00, 95% CI 0.92 – 1.09)
- Health-related quality of life: EQ-VAS (patient perception of outcome) (Scale 0-100, with 0 being the worst health, 100 being perfect health)
- Mean score 74 vs 75
- Adverse events:
- Higher rates of arrhythmias with haemodynamic instability in hypothermia group:
- 24% vs 16% (RR 1.45, 95% CI 1.21 – 1.75, p<0.001)
- Comparing hypothermia vs normothermia, no difference in:
-
- Pneumonia: 36% vs 35%
- Sepsis: 11% vs 9%
- Bleeding: 5% vs 5%
- Skin complications due to the device: 1% vs <1%
-
- Higher rates of arrhythmias with haemodynamic instability in hypothermia group:
Authors’ Conclusions
- In patients suffering out-of-hospital cardiac arrest, induced hypothermia did not lead to lower mortality
- Functional outcome and health related quality of life did not improve from hypothermia
Strengths
- This was an excellently conducted randomised trial with large numbers and a superb effort from all involved
- Design based on data achieved from multiple previous trials meaning a robust sample size calculation and estimate of absolute risk reduction
- Mortality in trial (50%) similar to that used in power calculations
- Excellent internal validity:
- Although 56% of those screened seems like a large proportion not to be enrolled, only 1.6% were for unknown reasons
- Allocation concealment and blinding of everyone involved except (understandably) treating clinicians
- Intention to treat analysis
- Clear separation between groups
- Near complete follow-up
- < 1% missing data on mortality
- 94% had functional outcome assessed by mRS
- Protocolised care minimised variability in practice
- Only 3 protocol deviations related to temperature management
- External validity high due to the multicentre and multinational nature of the study
Weaknesses
- Median temperature of 34°C in the hypothermia arm achieved at 3 hours after randomisation. Cooling was not implemented pre-hospital. It is logistically very difficult to achieve any earlier rapid cooling for many reasons (e.g. patients needing to go to cardiac catheter lab)
- The trial does not answer the question as to whether ultra-early cooling may be beneficial
- An ARR of 7.5% was an ambitious difference to achieve, particularly given the TTM results which showed no difference between 33°C and 36°C. However, the authors based this on the original 2002 trials
- It would be helpful to know exact numbers of patients in the normothermia group who were febrile. A graph is given in the supplementary index but it is difficult to give accurate numbers looking at this graph
- The trial does not guide our management of In-Hospital-Cardiac-Arrest, so generalisability to this cardiac arrest group is limited
- 20% of patients were co-enrolled in the TAME trial, 10% in each group. This could potentially be a confounder but this will not be known until the results of this trial are published
The Bottom Line
- I will aim for normothermia in my patients following out of hospital cardiac arrest. I will implement targeted temperature management if temperature exceeds 37.7°C
- Future exploration may include a trial of normothermia vs no targeted temperature management
External Links
- Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest
- Translating Targeted Temperature Management Trials into Postarrest Care
Metadata
Summary author: Celia Bradford @celiabradford
Summary date: June 17 2021
Peer-review editor: George Walker
Picture by: iStock




Really interesting outcome. So proactive TTM is as good as hypothermia and reactive TTM is safer than hypothermia, but I’m still wondering whether reactive TTM is better or worse than proactive TTM? I suppose there’s a cost factor and a safety factor if we can avoid invasive cooling devices in half our patients. So is everyone switching to follow TTM2 strategy until a direct comparison is investigated?
Thanks for this great summary Celia! I have a couple points which you might wish to comment on?
Your statement, “I will implement targeted temperature management if temperature exceeds 37.7°C” interested me as I presume you mean you will do this in preference to targeted hypothermia, rather than in preference to other methods of targeted normothermia such as that described in the TTM trial. Clearly TTM2 has not compared the two differing strategies for targeted normothermia, and therefore does not invalidate the TTM strategy.
I worry that the application of targeted temperature management in comatose survivors of cardiac arrest remains challenging, particularly outside the context of well-resourced multi-centre RCTs. Given the apparent importance of diligent avoidance of fever, perhaps some of the health system context can be informative? For example, even in this well-resourced trial in first-world health systems 55% of patients were not randomised due mostly to >180 minutes post-arrest. In addition, nearly 50% of patients in the normothermia group needed a cooling device and despite this 5% had temperature >38deg.
In my health service context I will argue that TTM2 does not invalidate the application of the learnings of the TTM trial, in fact it validates what we already know. All such comatose survivors of cardiac arrest should have cooling devices placed prophylactically, and fever avoided through the use of targeted temperature management.