Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia

Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia
van Baarle. NEJM 2023. doi: 10.1056/NEJMoa2214322
Clinical Question
- In haematology or ICU patients with platelet counts between 10,000 and 50,000 per mm3, is receiving no prophylactic platelets non inferior compared to the administration of 1u platelets in order to reduce the risk of catheter-related bleeding?
Background
- CVC placement and thrombocytopenia are both common among haematology and ICU patients
- Retrospective studies have suggested that with USS guidance CVC insertion is safe even with a platelet count < 20,000 per mm3
- Transfusion guidelines regarding platelet-count thresholds before the placement of a CVC offer conflicting recommendations regarding transfusion threshold:
- <20,000: British Society of Hematology
- <40 – 5000: American Society of Oncology
- <50,000: AAGBI for “other major surgery or invasive procedures”,
- Platelet transfusion is not without associated morbidity, alongside being a scarce and expensive resource
- Various adverse effects include TRALI, TACO, allergic reaction and alloimmunization
Design
- Randomised, open label, non-inferiority trial
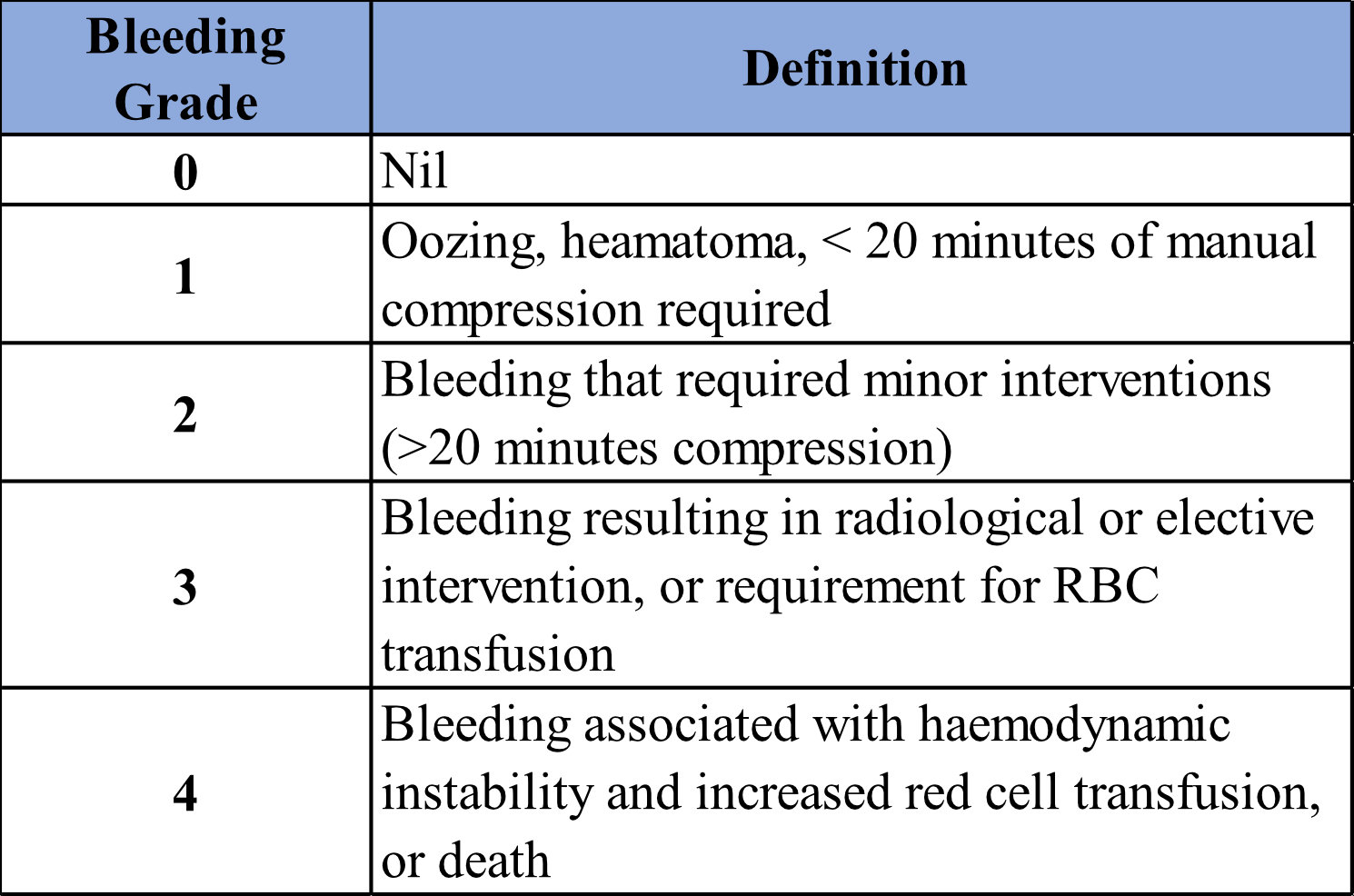
- Non-inferiority margin set as 2.5% increase in the absolute risk of Grade 2-4 bleeding
- i.e. if rate of bleeding was < 2.5% greater in no transfusion arm then this would be deemed non-inferior
- Occurrence of bleeding and any treatments recorded immediately post CVC placement and at 1- and 24-hours post insertion
- 1:1 randomization to transfusion vs no transfusion
- Stratified according to the trial centre and catheter type (large-bore dialysis catheter or regular CVC)
- Unblinded for patient but if possible the operator was unaware of the trial-group assignment
- Ideally informed consent taken prior to randomisation, but if emergency (ie. in ICU) then deferred consent allowed
- Sample size calculation required 196 CVC insertions in each group:
- Based on assumption that in transfusion group 1% will have Grade 2 bleeding and no patients will have Grade 3 or 4 bleeding
- 80% power to detect the non-inferiority of the non-transfusion strategy with an alpha level of 0.05
- No loss to follow-up accounted for
Setting
- 10 hospitals in the Netherlands (7 academic and 3 general)
- Included 8 hematology wards and 7 ICUs (Figure S2a)
- February 2016 – March 2022
Population
- Inclusion:
- Patients who had a platelet count of 10,000 to 50,000 per mm3
- within 24 hours before the procedure
- Exclusion:
- Therapeutic anticoagulation
- A history of congenital or acquired coagulation factor deficiency or bleeding risk
- INR>1.5
- Changed to INR > 3.0 during study due to new evidence
- Prior inclusion within 24 hours (the authors allowed multiple inclusions of patients who had been already included in the study, as long as each procedure was performed 24h apart.)
- Baseline Characteristics
- 411 CVC placements randomized –> 393 CVC placements (358 patients) were included in ITT analysis
- 18 excluded due to withdrawal or no deferred consent
- No major difference in baseline characteristics:
- Comparing transfusion vs no transfusion:
- Age: 58 vs 59
- Female: 34 vs 38%
- Median platelet count: 30,000 vs 30,000
- Median INR: 1.1 vs 1.1
- Median APTT: 29 vs 31
- ICU: 43 vs 44%
- Regular CVC (as opposed to dialysis catheter): 82 vs 84%
- Tunneled: 11 vs 10%
- IJ: 50 vs 50%
- Subclavian 38 vs 38%
- Prior platelet transfusion < 6 hrs before randomisation: 9 vs 10%
- 411 CVC placements randomized –> 393 CVC placements (358 patients) were included in ITT analysis
Intervention
- Transfusion 1u platelets prophylactically
Control
- No transfusion
Management common to both groups
- Ultrasound guided CVC
- Operator needed to have performed at least 50 CVC placements
- If possible, all catheters were placed approximately 1h after randomization
- Otherwise, CVCs were placed according to local clinical practice
Outcome
- Primary outcome:
- Grade 2-4 catheter-related bleeding:
- Transfusion 4.8% (transfusion) vs 11.9% (no transfusion)
- Absolute risk reduction of 7.1% (90% CI: 1.3 to 17.8%), RR 2.45 (90% CI 1.27 – 4.70)
- Grade 2-4 catheter-related bleeding:
- Selected secondary outcomes:
- Comparing transfusion vs no transfusion
- Significant difference in transfusion group in:
- Platelet count at 1h and 24h after CVC placement
- 54,000 vs 26,000 after 1h; 36,000 vs 26,000 after 24h
- Median ICU LOS
- 9 vs 7 days
- Platelet count at 1h and 24h after CVC placement
- No significant difference in:
- The risk of grade 3-4 catheter-related bleeding (2.1% vs 4.9%)
- Hematoma occurrence (12.2 % vs 18.9%)
- Rate of red-cell transfusion in ≤24 hr (0.48 vs 0.49)
- Allergic transfusion reaction (1 vs 0.5%)
- ICU mortality (57 vs 52%)
- Hospital mortality (28 vs 32%)
- Subgroup and Other Outcomes
- Exploratory subgroup analysis favoured transfusion group if patient had:
- Non-tunneled catheter
- Subclavian vein
- Haematology ward
- ICU patients has similar bleeding outcomes regardless of transfusion (4 vs 5%)
- Non-transfusion group received more platelet transfusions in the 24h after CVC placement
- Rate of platelet transfusion in 24 hours after CVC placement by pre-CVC platelet count (transfusion vs no-transfusion, per-protocol analysis)
- Platelet count 10-19: 0.19 vs 0.67
- Platelet count 20-29: 0.12 vs 0.58
- Platelet count 30-39: 0.11 vs 0.35
- Platelet count 40-50: 0.15 vs 0.16
- Rate of platelet transfusion in 24 hours after CVC placement by pre-CVC platelet count (transfusion vs no-transfusion, per-protocol analysis)
- Overall costs related to transfusion and bleeding events were higher in the transfusion group (total cost difference $410, 95% CI 285 – 545)
- Exploratory subgroup analysis favoured transfusion group if patient had:
Authors’ Conclusions
- In patients with severe thrombocytopenia, withholding prophylactic platelet transfusion before CVC placement in those with a platelet count of 10,000 to 50,000 mm3 did not meet the predefined margin for non-inferiority, and resulted in more CVC-related bleeding than prophylactic platelet transfusion
Strengths
- Multi-centre RCT
- Trial protocol and statistical analysis plans were published prior to publication
- ITT analysis
- Population is generalizable to our daily ICU patients in terms of background comorbidities and acute physiology
- Pragmatic and included a wide range of CVC insertions:
- Both tunneled and non-tunneled catheters
- Location of insertion
- Dialysis catheter vs standard catheters
- Inclusion of cost analysis
- Low rates of protocol violations
Weaknesses
- Single country may limit external validity
- Given operators aware that this trial was about rates of bleeding complications could lead to Hawthorne effect when comparing these bleeding rates to everyday practice
- Patient heterogeneity:
- 1 unit of platelets may not have been sufficient in all patients
- Is the bleeding risk the same in a general ICU patient compared to haematology patient?
- Bleeding risk in this study appears to be higher in hematology subgroup compared to ICU
- Potential for confounders – Figure S2b suggests that a large proportion of bleeding in no transfusion group occurred in 2 haematology centres (14/22 of all Grade 2 – 4 bleeding episodes).
- Missing data:
- 7% of the population did not have the primary outcome data available
- Not fully blinded:
- ~25% of insertions were unblinded to operator (Figure S7); however, analysis showed no increased rates of bleeding in unblinded group
The Bottom Line
- Alongside paying meticulous attention to insertion technique and the high rates of post CVC platelet transfusion in the no transfusion group, I will prophylactically transfuse if the platelet count is < 50,000 per mm3
External Links
Metadata
Summary author: Ryo Ueno
Summary date: 14th July 2023
Peer-review editor: George Walker
Picture by: Thavis3D /Unsplash



