65 Trial

Effect of Reduced Exposure to Vasopressors on 90-day mortality in Older Critically Ill Patients With Vasodilatory Hypotension: a randomised clinical trial
Lamontagne F, JAMA 2020. Published online February 12. doi:10.1001/jama.2020.0930
Clinical Question
- In patients older than 65-years with vasodilatory hypotension receiving vasopressors, does a target mean arterial pressure (MAP) of 60-65mmHg compared with usual care impact mortality?
Background
- The surviving sepsis campaign guidelines recommends targeting a MAP of at least 65mmHg in patients with septic shock. This recommendation has been controversial as it is based on expert opinion rather than evidence from clinical trials
- Protocolised management of sepsis and septic shock was made popular by Rivers in his landmark trial “Early goal-directed therapy in the treatment of severe sepsis and septic shock” published in NEJM in 2001. Whilst this trial had several methodological flaws, it lead the way for other large sepsis trials such as the ARISE trial, ProMise and Process which de-emphasised in their conclusions certain aspects of the Rivers trial, such as haemoglobin and ScVO2 targets. These trials did not give firm conclusions on MAP targets
- Sepsis has a huge global burden with 11 million deaths worldwide per annum. It also represents a high health cost and most countries necessitate ICU admission if patients require vasopressor support. It is therefore an important area of research to optimise therapy based on sound research evidence
- Vasopressors are not without risk. They may reduce blood flow to vasoconstricted vascular beds (eg gut), effect cardiac and immune function. The sub-group of elderly patients in the SEPSISPAM trial had a higher mortality in those with higher MAP targets which led to the design and conduct of this trial
Design
- Pragmatic, open, multicentre, parallel group, randomised clinical trial
- 1:1 randomisation
- Allocation concealment achieved by a web-based randomisation system
- Stratification by site with variable sized permuted blocks
Setting
- 65 ICUs in the UK
- Patients were randomised between July 3rd 2017 and March 16th 2019
Population
- Inclusion:
- Patients older than 65 years
- <6 hours since commencing vasopressor infusion
- Patients were adequately fluid resuscitated
- Vasopressors were expected to continue for at least 6 hours more
- Exclusion:
- Contraindication to permissive hypotension
- Vasopressors being used solely for bleeding, cardiac failure, post-CABGs vasoplegia
- Treatment for brain or spinal cord injury
- Death perceived as imminent
- Previous enrolment in 65 trial
- 2600 patients were randomised. 2463 were included in the primary analysis (after 137 declined or withdrew consent)
- Baseline variables were well matched
- Patients or their next of kin provided consent. Deferred consent after randomisation was permitted
- Randomisation took place 24 hours a day, 7 days per week
- The sample size calculation assumed a 90-day mortality of 35% in the usual care group (allowing a 2.5% withdrawal or loss to follow-up). The sample size of 2600 had a 90% power to detect a 6% absolute risk reduction in mortality in the permissive hypotensive group.
- An interim analysis was conducted with pre-specified stopping rules in the case of harm or effectiveness
- Intention to treat analysis
- A p-value <0.05 was considered significant
- Baseline variables that were adjusted for included age, sex, chronic hypertension, chronic heart failure, atherosclerotic disease , dependence for ADLs, duration of vasopressors
Intervention
- Permissive Hypotension
- The permissive hypotensive group received vasopressors to target MAP of 60-65mmHg. MAP alarm limits were set on monitors to prompt dosing to be decreased or increased.
- Noradrenaline, vasopressin, terlipressin, phenylephrine, epinephrine, dopamine and metaraminol were considered as vasopressors
- The permissive hypotensive group received vasopressors to target MAP of 60-65mmHg. MAP alarm limits were set on monitors to prompt dosing to be decreased or increased.
Control
- Standard Care
- MAP targets were at the discretion of the treating ICU team
Management common to both groups
- Choice of vasopressor and other treatments were at the discretion of the treating team
- The allocated intervention was administered throughout the ICU stay
Outcome
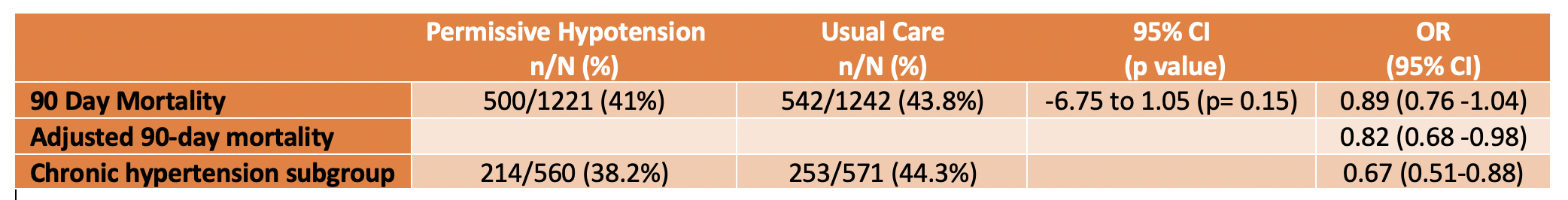
- Primary outcome: Mortality at Day 90 – no significant difference
- 500 (41%)/1221 in permissive hypotensive group vs 544 (43.8%)/1242 in usual care group (95% CI -6.75% to 1.05%; p=0.15)
- When adjusted for prespecified baseline variables, the OR for 90-day mortality was 0.82 (95% CI 0.68-0.98)
- The survival curves diverged early after randomisation peaking around 1 month and then staying apart from that point onward (up to 12 months)
- Secondary outcomes: no difference in any secondary outcome
- Mortality at ICU discharge and hospital discharge
- Duration of survival
- Duration of Respiratory support
- Duration of Renal support
- ICU LOS and Hospital LOS
- Cognitive decline at 90 days and 1 year
- Quality of life at 90 days and 1 year
- Subgroup analysis: the reduction in mortality was more pronounced in the permissive hypotension group patients who were identified to be chronically hypertensive and trended to greater survival the older you were
- Comparing permissive hypotensive group vs. standard care group
- Chronic hypertension subgroup – 90-day mortality 38.2% vs. 44.3% (adjusted OR 0.67, 95% C.I. 0.49-0.85)
- Patients without chronic hypertension – 90-day mortality:43.3% vs. 43.4%
- Comparing permissive hypotensive group vs. standard care group
- Vasopressor dose: Permissive hypotension vs usual care
- median dose: 17.7mg vs 26.4mg (noradrenaline equivalent)
- median duration: 33 hours vs 38 hours (difference -5 hours, 95% CI -7.8 to -2.2)
- MAP data
- There was a clear divergence between groups with mean and peak MAP values lower in the permissive hypotension group
- Mean: 66.7 vs. 72.6
- Peak: 83 vs. 92
- There was a clear divergence between groups with mean and peak MAP values lower in the permissive hypotension group
- Co-interventions: corticosteroid use, fluid balance and urine output were similar between the intervention and control arm
- Serious adverse events were similar between the two groups
Authors’ Conclusions
- In patients older than 65 years receiving vasopressors for vasodilatory shock there was no difference in 90 day mortality but the point estimate for the primary outcome favours permissive hypotension
Strengths
- Allocation concealment, intention to treat analysis, near complete follow-up
- Good internal and external validity
- Large multi-centre trial in diverse ICU patient group
- Clear divergence in MAP achieved
- Rapid completion of trial with recruitment exceeding aims
- Clinically significant outcomes assessed including cognition and quality of life
- In contrast to the SEPSISPAM trial, patients with chronic hypertension in this trial were not more likely to develop renal dysfunction compared to the usual care group
Weaknesses
- Unblinded: the usual care group did not have a prescriptive MAP target, however, there was a clear separation in MAP achieved
- Interpretation of subgroups should be cautious (eg the group with chronic hypertension and the very elderly, as the trial was not powered to detect differences in the subgroups)
- With a difference in mean MAP of only 5mmHg between the intervention and the control group, it is likely that a larger trial would be required to detect a significant mortality difference, if there is one
The Bottom Line
- It appears safe to target a MAP of 60-65mmHg in elderly patients with vasodilatory shock and I would not hesitate to apply this to my patients
- There is a suggestion of a potential mortality benefit with the use of permissive hypotension in patients that have chronic hypertension. Further trials are needed to test this hypothesis
- I look forward to a meta-analysis and potentially an update in the surviving sepsis campaign guidelines
External Links
Metadata
Summary author: Celia Bradford – @celiabradford
Summary date: 13th February 2020
Peer-review editor: Dave Slessor




just a heads up – I think the video link to vimeo presentation at #CCR20 is broken.
Thanks Jon – appreciate you letting us know 🙂 Should be working now
good analysis but what is the biological rationale behaind having lower MAP target for elderly chronic hypertensives? we all have learnt about the shift of autoregulation of blood pressure in these patients. The study observation is likely to be by chance. Isnt it unsafe to apply these to our subset of patients?
Video link doesn’t work. Link Here:
https://vimeo.com/383967830
Updated – thanks David!