APROCCHSS

Hydrocortisone plus Fludrocortisone for Adults with Septic Shock
Annane. NEJM 2018; 378: 809-818. DOI: 10.1056/NEJMoa1705716
Clinical Question
- In critically ill patients with septic shock, does the combination of hydrocortisone plus fludrocortisone therapy reduce 90 day mortality?
Background
- The use of steroids in critically ill patients continues to be controversial. Whilst there are signals for improved cardiovascular parameters, this did not translate to clear mortality benefits.
- The most recent of these trials (ADRENAL, 2018), concluded that among patients with septic shock undergoing mechanical ventilation, a continuous infusion of hydrocortisone did not result in lower 90-day mortality than placebo.
Design
- Multi-centre
- Randomised placebo controlled trial
- Double blinded
- Initially designed to have 4 parallel groups to evaluate benefits/risks of steroids and drotrecogin alfa (DAA) in a 2 by 2 factorial design
- Original 4 groups
- Group 1: Corticosteroids placebo + DAA placebo
- Group 2: Corticosteroids + DAA placebo
- Group 3: Corticosteroids placebo + DAA
- Group 4: Corticosteroids + DAA
- When drotrecogin alfa was withdrawn from the market, the trial was continued with 2 parallel groups
- Group 1 and 3 combined
- Group 2 and 4 combined
- Original 4 groups
- Randomised by permuted blocks of 8
- Intention to treat analysis
- Sample size calculation
- Based on anticipated 90 day mortality of 45% among pts with septic shock
- 320 pts required in each of the original 4 groups (1280 pts total) for 95% power to detect an absolute difference of 10% in 90 day mortality (α=0.05)
Setting
- 34 French ICUs
- September 2008 – June 2015
- Trial suspended twice: October 2011 – May 2012 (DAA withdrawal) and July 2014 – October 2014 (request of data and safety monitoring board to check the quality of the trial agents and the distribution of the serious adverse events)
- 1671 patients screened; 1241 randomised into 4 groups
Population
- Inclusion: Intensive care patients with indisputable or probable septic shock for less than 24 hours
- Septic shocked defined as clinically or microbiologically documented infection
- SOFA score of 3 or 4 for at least 2 organs and at least 6 hours in duration
- Vasopressor therapy for at least 6 hours to maintain a systolic blood pressure of at least 90mmHg or a mean blood pressure of 65mmHg
- Exclusion:
- Presence of septic shock for more than 24 hours
- High risk of bleeding
- Pregnancy or lactation
- Underlying condition which would limit short-term survival
- Known hypersensitivity to drotrecogin alfa (later removed)
- Previous treatment with corticosteroids
- Baseline characteristics were similar: intervention vs placebo group
- Mean age (years): 66 vs 66
- Male sex (%): 65.5 vs 67.7
- Medical admission (%): 82.4 vs 81
- SOFA score: 12 vs 11
- Site of infection (%)
- Unknown: 1.8 vs 2.9
- Lung: 60.7 vs 58.0
- Abdomen: 12.1 vs 10.9
- Urinary tract: 16.6 vs 18.8
- Positive blood culture (%): 36.6 vs 36.6
- Vasopressor administration
- Epinephrine
- Number of pts: 53 vs 58
- Dose (mcg/kg/min): 2.31 vs 1.74
- Norepinephrine
- Number of pts: 534 vs 552
- Dose (mcg/kg/min): 1.02 vs 1.14
- Epinephrine
- Mechanical ventilation (%): 92.3 vs 91.3
- RRT (%): 27.0 vs 28.1
- % of pts who received DAA: 17.1 (105/614) vs 16.4 (103/627)
Intervention
- Hydrocortisone
- 50mg IV bolus every 6 hours
- Fludrocortisone
- 50 mcg tablet once in the morning
- Administered for 7 days without tapering
Control
- Placebo
- Similar in appearance and manufactured for the trial
Management common to both groups
- Before randomisation, plasma total cortisol levels measured before, 30 and 60 minutes after IV bolus of 250 mcg of corticotrophin (Synacthen)
- Other interventions were harmonised across centres according to 2008 Surviving Sepsis Campaign guidelines
- National guidelines for the prevention of superinfection were followed
Outcome
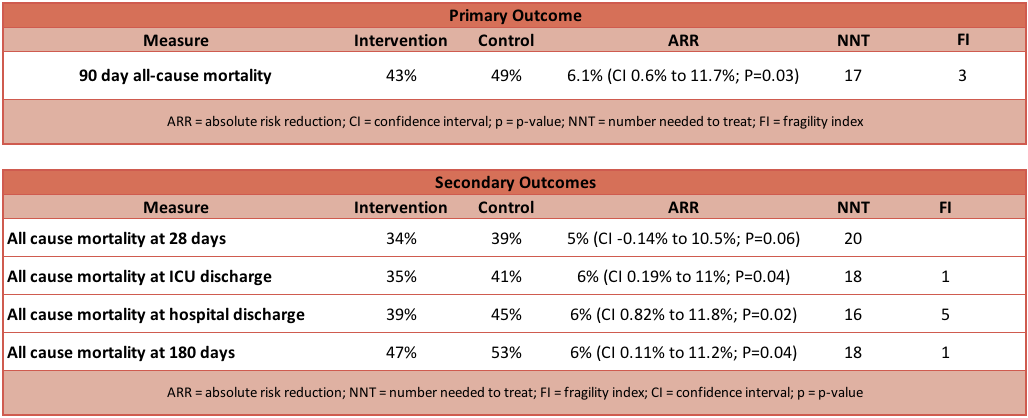
- Primary outcome: Significant reduction in 90 day mortality in intervention compared to control group
- Intervention group: 264 of 614 (43.0%) patients had died
- Control group: 308 of 627 (49.1%) patients had died
- Relative Risk (RR): 0.88 (95% CI 0.78 to 0.99; P=0.03)
- Absolute Risk Reduction (ARR): 6.1% (95% CI 0.6% to 11.7%; P=0.03)
- Number Needed to Treat (NNT): 17
- Fragility Index (FI): 3
- Secondary outcome: Intervention vs control group
- Significantly in favour of intervention group
- All cause mortality at ICU discharge
- 35% vs 41% (RR 0.86; 95% CI 0.75–0.99; P=0.04)
- All cause mortality at hospital discharge
- 39% vs 45% (RR 0.86; 95% CI 0.76–0.98; P=0.02)
- All cause mortality at 180 days
- 47% vs 52% (RR 0.89; 95% CI 0.79–0.99; P=0.04)
- No of days that pts were alive and free from vasopressors up to 28 days
- mean 17+/-11 vs 15+/-11, P<0.001
- Organ-failure-free days up to day 28
- mean 14+/-11 vs 12+/-11, P=0.003
- % of pts weaned from vasopressors at 28 days
- P<0.001
- % of pts weaned from mechanical ventilation at 28 days
- P=0.006
- % of pts with SOFA score below 6 at day 28
- P<0.001
- All cause mortality at ICU discharge
- No significant difference between groups
- All cause mortality at 28 days
- 34% vs 39% (RR 0.87; 95% CI 0.75–1.01; P=0.06)
- % of pts from whom care was withheld or withdrawn
- 10.4% vs 9.7%, P=0.69
- No of days that pts were alive and free from mechanical ventilation up to 28 days
- mean 11+/-11 vs 10+/-11, P=0.07
- Safety outcomes/Incidence of serious adverse events
- 53.1% vs 58%, P=0.08
- All cause mortality at 28 days
- Significantly in favour of intervention group
Authors’ Conclusions
- In critically ill patients with septic shock, the addition of hydrocortisone and fludrocortisone compared to placebo was associated with a significant improvement in mortality at 90 days.
Strengths
- Randomised controlled trial
- Multi-centre
- Intention to treat analysis
- Appropriate primary outcome
- Appropriateness of antibiotic therapy recorded
Weaknesses
- The trial was initially designed and powered with DAA being part of the therapy. The withdrawal of DAA has impacted aspects of this trial including statistical power calculation
- Statistical analysis published in supplementary material suggest no interaction with DAA and other treatments
- The Fragility Index for several of the outcomes (including primary outcome) in favour of intervention is in single figures
- Outcome of Synacthen test conducted pre-randomisation was not mentioned or discussed in main paper
- Results discussed in supplementary material section where there was no difference between responders and non-responders in those from which the Synacthen test was actually conducted
- The trial was conducted using the Surviving Sepsis Guidelines from 2008 which has since been updated
- Very sick patient population – the high doses of vasopressors used in the trial population may limit external validity
- Not all secondary endpoints included in original trial protocol reported on in final manuscript
The Bottom Line
- The addition of fludrocortisone and its effect is less well investigated compared to hydrocortisone by itself. It is not my current practice to administer this drug for refractory septic shock
- Primary and secondary outcomes, including safety profile, shows a trend in favour of the corticosteroid group (consistent with the findings of the ADRENAL trial)
- I will continue my current practice of using hydrocortisone IV (6 hourly) for refractory septic shock
External Links
- [article] Hydrocortisone plus Fludrocortisone for Adults with Septic Shock
- [further reading] ADRENAL trial review
- [further reading] TBL Steroid Review
Metadata
Summary author: Adrian Wong
Summary date: 15 March 2018
Peer-review editor: Segun Olusanya



