AVB-TIPS
Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis
Lv. Lancet Gastroenterology Hepatology 2019; published online 29 May 2019. doi:10.1016/S2468-1253(19)30090-1
Clinical Question
- In patients with cirrhosis and acute variceal bleeding, does early transjugular intrahepatic portosystemic shunt (TIPS) compared to standard care increase transplant-free survival?
Background
- Acute variceal bleeding, associated with advanced liver cirrhosis, has a poor prognosis with 6-week mortality as high as 55%
- Standard care involves vasoactive drugs, prophylactic antibiotics, endoscopic therapies and organ support
- When standard care has failed, TIPS procedures can be performed to reduce porto-venous pressures at the cost of increased encephalopathy
- Early TIPS have been advocated but previous trials have been criticised for excessive bias from poor methodology, inappropriate outcome measures or under-powered sample sizes
Design
- Single-centre randomised, controlled trial
- Written informed consent from all patients or their next of kin
- Randomised 2:1 ratio
- Manuscript justifies this ratio by stating “on the basis of previous studies that showed early TIPS improved survival … and to encourage recruitment”
- Performed through web-tool with adequate concealment of random sequence
- Dynamically balanced using Pocock and Simon’s minimisation method, balanced for:
- Child Pugh class B vs C
- Presence or absence of active bleeding
- Clinicians and patients were not blinded to allocation due to the nature of the intervention
- Outcome assessors and investigators remained unaware of group allocation
- Target sample size was 120 assuming:
- Power 80% and alpha significance level 0.05
- Baseline survival in Standard Care group 60%
- Absolute survival increase of 25% (i.e. survival in Early TIPS group of 85% – this would equate NNT 4)
- Drop-out rate 5%
- Modified Intention-to-treat analysis after removing patients that were randomised but did not meet inclusion criteria
- Supplemented by per-protocol analysis
- Pre-specified and post-hoc subgroup analyses performed
- Primary analysis performed using probability curve estimates (Kaplan-Meier), which look at the estimated rate of events occurring over time (rather than a simple count of events that have occurred at a specific follow-up time)
- Each estimate is expressed as a Hazard Rate and the comparison of two Hazard Rates is expressed as the Hazard Ratio
- This increases the power of correctly rejecting the null hypothesis (a true positive conclusion) despite a smaller sample size
Setting
- Single centre in tertiary university hospital in China
- June 2011 to Sept 2017
Population
- Inclusion: Adults between 18 and 75 years of age; liver cirrhosis; endoscopically proven acute variceal bleeding; Child-Pugh B or C
- Exclusion: Uncontrolled bleeding prior to randomisation; bleeding from isolated gastric or ectopic varices; severe cardiopulmonary disease; spontaneous recurrent hepatic encephalopathy; complete portal vein thrombosis; creatinine greater than 3 mg/dL (265 umol/L); malignancy; uncontrolled infection or sepsis; previous shunt (TIPS or surgical procedure) or previous standard care (beta-blockade and variceal banding); contra-indications to TIPS; pregnancy; unable to give consent or declined consent
- 373 screened; 132 randomised (TIPS = 86; Standard Care = 46)
- 3 excluded after randomisation as did not meet inclusion/exclusion criteria
- Baseline demographics were similar between groups (Early TIPS vs Standard Care)
- Mean age: 50 vs 50 years
- Gender: 63% vs 76% male
- Cirrhosis aetiology:
- Hep B Virus: 74% vs 76%
- Hep C Virus: 4% vs 9%
- Alcohol: 2% vs 9%
- Autoimmune: 4% vs 2%
- Primary biliary cholangitis: 5% vs 0%
- Unknown: 12% vs 4%
- Mean MELD score: 14.0 vs 13.4
- Mean Child-Pugh score: 8.0 vs 8.0
Intervention
- Early TIPS
- Transjugular Intrahepatic Portosystemic Shunt performed within 72 hours (preferably within 24 hours) of initial diagnostic endoscopy
- Vasoactive drugs continued until TIPS performed
- Prophylactic antibiotics given for 5–7 days from admission
- TIPS performed under conscious sedation and with local anaesthetic at the puncture site
- 8 mm covered stent inserted and dilated to 8 mm
- If portal hypertension recurred or ultrasound demonstrated shunt dysfunction, a TIPS revision with angioplasty or replacement stent was performed
Control
- Standard Care
- Vasoactive drugs continued from admission up to day 5
- Propranolol started on day 6 at 20 mg twice daily
- Titrated to reduce resting heart rate by 25% but not lower than 55 bpm
- Planned endoscopic band ligation was performed within 7–14 days after initial diagnostic endoscopy
- Repeated approximately every 14 days until varices were gone
- Monitoring endoscopy was performed every six months
- TIPS were inserted if bleeding failed to be controlled or clinically significant bleeding occurred
Management common to both groups
- Initial bleeding episode was controlled by vasoactive drugs and endoscopic therapy in both groups
- Vasoactive drugs: octreotide, somatostatin, terlipressin
- Endoscopic therapy: banding, sclerotherapy
Outcome
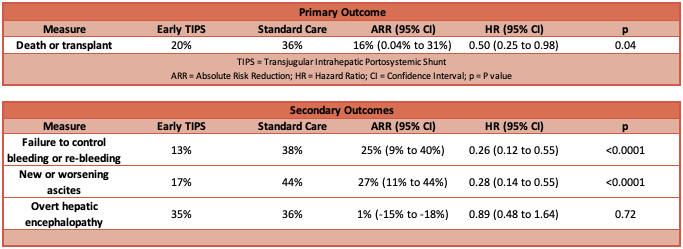
- Primary outcome: transplant-free survival was greater in the Early TIPS group
- Survival estimates model
- Hazard Ratio: 0.50 (95% CI 0.25 to 0.98; P = 0.04)
- This means the average event rate (death or transplant) during follow-up was half in the Early TIPS group compared to the Standard Care group
- The wide 95% CI means that the true effect probably lies somewhere between an event rate in the Early TIPS group of one-quarter that of the Standard Care group (clinically a very significant difference) or almost equal (no effect difference from Early TIPS)
- Absolute Risk Difference: -16% (95% CI -31% to 0.04%)
- Number Needed to Treat: 7
- Hazard Ratio: 0.50 (95% CI 0.25 to 0.98; P = 0.04)
- Incidence of death or transplant by last follow-up :
- Early TIPS group: 20%
- Standard Care group: 36%
- Absolute Risk Reduction (ARR): 15.48% (95% CI -0.55% to 31.50%; P = 0.0529)
- Fragility Index: 0
- This time-point analysis has used Fishers’ Exact test
- This biostatistical method has a lower power of identifying a true positive result than the event-rate analysis used by the trial authors
- A Fragility Index of zero indicates that the conventional statistical significance threshold is not met by simply using an alternative biostatistical method, and illustrates that the strength of the conclusion from this trial is not very robust
- Incidence of liver transplant by last follow-up:
- Early TIPS group: 2%
- Standard Care group: 2%
- Hazard Ratio: 0.87 (95% CI 0.08 to 9.57; P = 0.91)
- Authors postulate that Chinese policy and availability of organs for transplant reduced the incidence of transplant
- Survival estimates model
- Secondary outcome:
- Failure to control bleeding or re-bleeding occurred less in the Early TIPS group
- New or worsening ascites occurred less in the Early TIPS group
- Overt hepatic encephalopathy was not different between groups
- Other complications and adverse events were not different between groups
Authors’ Conclusions
- In patients with advanced liver cirrhosis and acute variceal bleeding, early TIPS is superior to standard care (vasoactive drugs and endoscopic therapy)
Strengths
- Important question in high-risk and high-occurrence population
- Clearly postulated hypothesis and reasonable methodology to test this
- Good biostatistical methods using event-rate analysis rather than time-point analysis
- Adequate concealment of randomisation sequence
- Blinding of assessors and investigators
- Good period of follow-up (median two years)
- Measured benefits and possible harm from intervention
Weaknesses
- Unclear why 2:1 ratio used
- If there was the belief that early TIPS was best, then was there equipoise to ethically conduct the trial?
- Impossible to blind clinician and patient, possibly introducing bias that usually favours the intervention group
- The result is Fragile
- The null hypothesis is not rejected when a more conventional, albeit weaker, statistical analysis is performed
- This indicates that this trial is probably under-powered, that the precision of the estimated effect size is poor (wide confidence interval) and the possibility of the result actually being a false positive is higher than would be ideal
- Single-centre study from China reduces external validity (generalisability)
- The authors note differences in porto-caval pressure gradients before and after TIPS compared to a similar European trial and suggest that ethnicity is relevant
- The majority of patients had cirrhosis due to viral disease, with a minority due to alcohol consumption
- This may not be representative of other populations (certainly not my local population!) and therefore the result may not be generalisable
The Bottom Line
- This well conducted trial demonstrated that early TIPS was associated with a reduction in death or transplant events, and the conclusion is probably correct
- The limitations of this trial make the strength of the conclusion weak and impossible to generalise to non-Chinese populations (and probably to non-viral cirrhosis patients too)
- The hypothesis should be tested again in a broader population with a greater sample size
- In the meantime, I will engage with my hepatology / endoscopy team early to discuss ideal management including TIPS for each individual patient
External Links
- [article] Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis
- [further reading] Biostatistics Primer: What a Clinician Ought to Know: Hazard Ratios
- [further reading] TIPS on LITFL
Metadata
Summary author: Duncan Chambler
Summary date: 17 July 2019
Peer-review editor: David Slessor



