BALANCE – 7 vs 14 days of antibiotics
Antibiotic Treatment for 7 versus 14 Days in Patients with Bloodstream Infections
BALANCE Investigators. NEJM 2024; DOI: 10.1056/NEJMoa2404991
Clinical Question
- In hospitalised patients with bloodstream infections (BSI), is antibiotic treatment for 7 days, compared to treatment for 14 days, non-inferior with respect to mortality at 90 days?
Background
- BSI are common and a leading cause of mortality and morbidity, accounting for 2.9 million deaths per year worldwide
- Early appropriate antibiotic therapy improves survival, but there are limited studies assessing duration of antibiotic therapy
- Shorter duration of therapy would confer benefits such as decreased antimicrobial exposure, complications, antimicrobial resistance, and costs
- Conversely, insufficient duration of antibiotic therapy could result in treatment failure, relapse, or selection of resistance
- Prior trials comparing antibiotic duration:
- Yahav et al. 2019 – Individualised, CRP-guided or 7 days fixed duration non-inferior compared with 14 days for gram-negative BSI
- von Dach et al. 2020 – 7 days non-inferior to 14 days in uncomplicated gram-negative BSI
- Molina et al. 2022 – 7 days is non-inferior to 14 days antibiotic duration for Enterbacterales BSI
Design
- Multicentre, investigator-initiated, open-label, randomised, non-inferiority trial
- Whilst open-label, allocation of antibiotic treatment group concealed until day 7 of adequate antibiotic therapy
- Initially only for ICU patients but extended to include all hospital patients after successful parallel trial of ward patients
- Eligible patients were assigned (1:1) via web-based randomization with variable block sizes, stratified according to hospital site and whether admitted to the ICU or hospital ward
- Sample size 3626 based on non-inferiority margin of 4% (assuming a baseline 90-day mortality of 22%) and accounting for maximum 5% loss to follow-up would achieve 80% power, at a one-sided alpha level of 2.5%
- Primarily intention-to-treat analysis, however modified intention to treat and per-protocol analysis also conducted
- Modified intention-to-treat analysis to exclude patients who died before day-7 of treatment (where the groups diverge).
- Per-protocol excluded all those who had more than a 2-day difference (+ or -) in assigned duration
- Daily assessment of adherence and reason for non adherence reported
- Pre-specified subgroup analyses based on source of infection, location of enrolment (ICU vs not), gram stain, vasopressor use and APACHE II score
- Trial protocol published a priori
- Informed consent obtained from patients or substitute decision maker prior to enrolment
Setting
- Conducted in 74 hospital sites across 7 countries
- Canada, Australia, New Zealand, Saudi Arabia, Israel, Switzerland, United States
- Oct 17, 2014 to May 5, 2023
Population
- Inclusion:
- Adult patients admitted to hospital with a positive blood culture with a pathogenic bacterium
- Exclusion:
- Severely immunocompromised (i.e. absolute neutrophil count < 0.5×10^9/L, or receiving immunosuppressive therapy for solid organ or bone marrow or stem cell transplant)
- Prosthetic heart valves or endovascular grafts
- Documented or suspected infectious syndrome for which prolonged antibiotics treatment was necessary (i.e. endocarditis, osteomyelitis, septic arthritis, undrained abscess, unremoved prosthesis-associated infection)
- Blood culture positive with a common contaminant (e.g. Coagulase-negative Staphylococci)
- Staphylococcus aureus or Staphylococcus lugdunensis bacteraemia
- Bacteraemia from rare organisms requiring prolonged treatment
- Fungaemia
- 36637 assessed for eligibility → 13,597 eligible → 3631 patients randomised
- 1824 patients assigned to 7-day group, and 1807 patients to 14-day group
- Comparing baseline characteristics of 7 day vs. 14 day group
- Male sex: 54% vs 53%
- Median age (yrs): 70 vs 70
- Median SOFA score day 0: 4 vs 5
- Enrolled in ICU: 55% vs 55%
- Mechanical ventilation: 21% vs 22%
- Coexisting conditions:
- Diabetes mellitus: 33% vs 31%
- Solid organ cancer: 22% vs 21%
- Glucocorticoid or immunosuppressant: 13% vs 12%
- Source control procedure: 44% vs 46%
- Source of bacteraemia
- UTI: 42% vs 43%
- Intra-abdominal or hepatobiliary: 19% vs 19%
- Lung: 13% vs 13%
- Vascular catheter: 6% vs 6%
- Skin, soft tissues, both: 6% vs 5%
- Other: 2% vs 2%
- Unidentified: 13% vs 12%
- Most commonly isolated pathogen
- Ecoli: 44% vs 43%
- Klebsiella spp.: 15% vs 16%
- Enterococcus spp.: 7% vs 7%
Intervention
- 7 days adequate antibiotic therapy
- Median duration received 8 days
Control
- 14 days adequate antibiotic therapy
- Median duration received 14 days
Management common to both groups
- Selection of antibiotics, duration and route was at discretion of treating clinician
- Adequate antibiotics was defined as per local laboratory susceptibilities
Outcome
- Primary outcome:
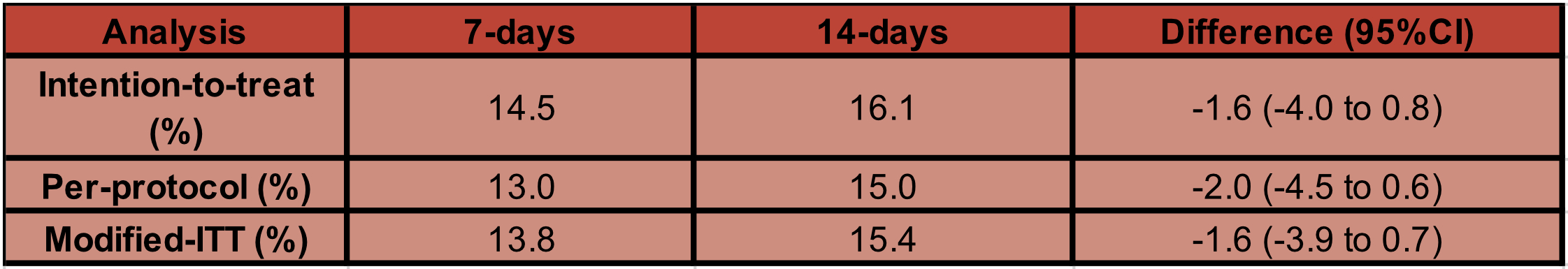
- Death from any cause at 90 days from the date of collection of index positive blood culture
- 7-day group was non-inferior
- Difference: -1.6% (95% CI, -4.0 to 0.8)
- No difference in pre-specified subgroups
- Secondary outcomes:
- Comparing 7 vs 14 days
- Significant difference in:
- Median hospital length of stay (days): 10 vs 11
- Difference: −1 days (95% CI, −1.5 to −0.5)
- Median hospital-free days by day 28: 17 vs 15
- Difference: 2 days (95% CI, 0.8 to 3.2)
- Median number of antibiotic free days by day 28: 19 vs 14
- Difference: 5 days (95% CI, 4.6 to 5.4)
- Median hospital length of stay (days): 10 vs 11
- No significant difference in:
- Hospital mortality: 9.3% vs 10.3%
- Median ICU length of stay (days): 5 vs 5
- Median days of vasopressor use: 3 vs 3
- Median days on mechanical ventilation: 6 vs 5
- Relapse rates of bacteraemia with same organism: 2.6 vs 2.2%
- C difficile infection: 1.7 vs 2.0 %
- Secondary infection/colonisation with antimicrobial-resistant organisms: 9.5 vs 8.5%
Authors’ Conclusions
Among hospitalized patients with bloodstream infection, antibiotic treatment for 7 days was noninferior to treatment for 14 days
Strengths
- Pragmatic design with patient centred outcomes:
- Inclusion of ward patients increases generalisability; conversely the inclusion of ward patients may make the overall cohort more heterogenous and less unwell
- Multicentre, international RCT
- Largest trial looking at antibiotic duration and included wide variety of pathogenic bacteria
- However some common bacteria not included such as Staph aureus, which represents a large proportion of BSI with high morbidity and mortality
- Minimal attrition: 0.5% withdrew consent (7 in intervention group, 10 in control group), 0.7% lost to follow-up (12 in intervention group, 15 in control group).
- Similar baseline characteristics, disease severity and frailty scores
- Intention-to-treat analysis supported by per-protocol and modified-ITT analysis to mitigate non-adherence/protocol deviation
- Clinicians blinded to allocation up until 7 days before being instructed to continue or cease antibiotics to avoid influencing antibiotic choices and clinical decision-making
- Objective primary outcome measures partly mitigates open-label design
- Central adjudication committee blinded to treatment allocation to look at secondary outcome such as relapse and secondary infection/colonisation
Weaknesses
- Predominantly Canadian hospitals (75%, 2712/3608) may not be representative of all practices
- Whilst 4% non-inferiority margin is lower than other similar trials and ultimately the point estimate favoured the 7-day group, given antimicrobials are one of the most important tools to treat patients within ICU some may view a 4% margin as too high for a non-inferiority trial
- Not powered to examine whether some subgroups benefit from prolonged durations such as differing sources of infection
- In particular, UTIs made up 40% of study population, one would expect clinical stability would be quickly achieved with appropriate antibiotics and extended antibiotic regimens would be uncommon for most
- Non-adherence rate was high
- 24% in 7-day group (23% received antibiotics for longer duration) and 17 % in 14-day group (including 6% receiving antibiotics for shorter duration and 11 % receiving antibiotics for longer duration)
- Per-protocol analysis similar to overall results
- It would be interesting to see the reasons for this
- No mention of whether there were patients who were already on antibiotics prior to index positive blood culture; and if they were, whether these would have ultimately treated the BSI
- Daily visits by research team could potentially introduce performance bias / Hawthorne effect
The Bottom Line
- This well-executed multi-centre trial provides solid evidence that a 7-day course of appropriate antibiotics is sufficient to treat most bloodstream infections, although it was not powered to examine different sources of BSI
- It highlights the importance of further research into antibiotic duration and antimicrobial stewardship
External Links
Metadata
Summary author: Tim Law
Summary date: 19th December 2024
Peer-review editor: George Walker
Picture by: Pixabay / Pexels




