BLING2
A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis
Dulhunty. Am J Respir Crit Care Med. First published online 22 Jul 2015. DOI: 10.1164/rccm.201505-0857OC
Clinical Question
- In adult ICU patients on beta-lactam antibiotics, does continuous infusion compared to intermittent bolus increase survival?
Design
- Randomised, controlled trial
- Double-blind with double-dummy preparations
- Unblinded staff prepared drugs and consecutively labelled sealed opaque envelopes
- Study participants, clinicians and data investigators remained blinded
- Pilot trial conducted to guide power calculation
- Sample size of 420 required to achieve 90% power to detect a 3 day difference in the primary outcome
- Intention-to-treat (ITT) analysis for primary end points, in addition to:
- Modified intention-to-treat (mITT) conducted in eligible patients who received a study drug
- A priori per protocol analysis in those who received 3 or more days of a study drug
Setting
- 25 Intensive Care Units (17 in Australia, 7 in New Zealand and 1 in Hong Kong)
- July 2012 to April 2014
Population
- Inclusion: adults with severe sepsis commenced on any of:
- Piperacillin-tazobactam
- Ticarcillin-clavulanate
- Meropenem
- Exclusion:
- Received beta-lactam for more than 24 hours duration prior to randomisation
- Pregnancy
- Allergy to study drugs
- No central venous line with >= 3 lumens
- Palliative treatment only, limits to care, death is imminent or death is likely within 90 days due to underlying process
- 2630 patients screened, 432 eligible patients were randomised (ITT), 422 received a study drug (mITT)
- Pathogen isolated in blood cultures in 83 patients
Intervention
- Continuous infusion of beta-lactam antibiotics
- Loading dose of chosen antibiotic
- Continuous infusion thereafter
- 1g in 100ml Meropenem over 24 hours
- 13.5g in 250ml Piperacillin-tazobactam over 24 hours
- 12.4g in 250ml Ticarcillin-clavulanate over 24 hours
- Administered with a normal saline dummy intermittent infusion
Control
- Intermittent infusion of beta-lactam antibiotics
- Loading dose of chosen antibiotic
- Intermittent infusion thereafter
- 1g in 20ml Meropenem once daily
- 4.5g in 20ml Piperacillin-tazobactam three times daily
- 3.1g in 20ml Ticarcillin-clavulanate four times daily
- Administered with a normal saline dummy continuous infusion
Management common to both groups
- Infusions were administered via a primed central venous line
- A change between the three beta-lactam antibiotics or to flucloxacillin was permitted within 14 days
- If flucloxacillin was introduced, a non-blinded loading dose was administered followed by blinded administration as per the patient’s allocation
- Total dose over 24 hours was the same regardless of group allocation
Outcome
- Characteristics of the groups (continuous vs intermittent)
- APACHE II score: 21 [IQR 17-26] vs 20 [IQR 16-25]
- Median antibiotic administration prior to randomisation: 13 hours vs 12 hours
- Median duration of blinded study drug treatment: 3.2 days [IQR 1.9-6.0] vs 3.7 days [IQR 1.9-5.9]
- Median total course of beta-lactam antibiotic: 5.3 days [IQR 2.9-7.7] vs 5.0 days [IQR 3.1-8.0]
- Change in antibiotic: 9.4% patients vs 11.8% patients
- Choice of antibiotic:
- Piperacillin-tazobactam: 69.3% vs 71.4%
- Meropenem: 29.7% vs 27.3%
- Ticarcillin-clavulanate: 0.9% vs 1.4%
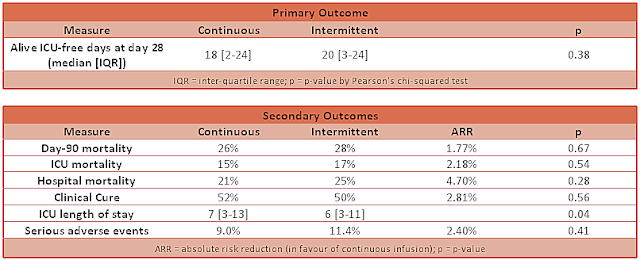
- Primary outcome: no difference was demonstrated in alive ICU-free days at 28 days
- Continuous group: 18 days [IQR 2-24]
- Intermittent: 20 days [IQR 3-24]
- P-value: 0.38
- Secondary outcome:
- ICU-free days at 90 days: no difference found
- Hazard ratio: 0.91 (95% CI 0.63-1.31; p-value = 0.61)
- Clinical cure at 14 days after antibiotic cessation: no difference found
- Odds ratio: 1.12 (95% CI 0.77-1.63; p-value 0.56)
- ICU-free days at 90 days: no difference found
- Modified Intention-to-treat and per-protocol analysis
- Differences remained non-significant for these two a priori defined populations
Authors’ Conclusions
- In a heterogenous Intensive Care Unit population, continuous and intermittent infusion of beta-lactams appear to have equivalent outcomes
Strengths
- Inclusion of a pragmatic, heterogenous ICU population including those on renal replacement therapy
- Appropriate randomisation, concealment and blinding
- Double-blind and double-dummy administration removes possible bias, which is particularly important given that the treating clinicians could influence the outcome measure (early or late discharge from ICU)
Weaknesses
- Not powered to detect mortality differences; this study used alive ICU-free days at 28 days as a surrogate
- Relatively short antibiotic courses were used, and so may not represent all ICU practice
- Very few patients received ticarcillin-clavulanate, so the results may not be representative for this antibiotic
- Clinical cure was assessed subjectively by blinded clinician
The Bottom Line
- In ICU patients, with underlying pathophysiological changes of sepsis and organ dysfunction, there appears to be no benefit from continuous infusion of beta-lactam antibiotics compared to intermittent bolus infusion
- Given the simplicity of an intermittent bolus infusion, this should be the preferred method for these antibiotics in this patient group
External Links
- [article] A multicenter randomized trial of continuous versus intermittent beta-lactam infusions in severe sepsis
- [further reading] Alternative dosing strategies for intravenous antibiotics to treat severe infections
- [further reading] LITFL CCC – Antimicrobial dosing and kill characteristics
Metadata
Summary author: @DuncanChambler
Summary date: 2 October 2015
Peer-review editor: @avkwong