COV-BARRIER

Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19: a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial
Marconi V et al. Lancet Respir Med 2021, Published online September 1 2021, https://doi.org/10.1016/S2213-2600(21)00331-3
Clinical Question
- In hospitalised adult patient with COVID-19, does baricitinib compared with placebo decrease a composite outcome of progression of illness and mortality?
Background
- Since January 2020, more than 4.5 million people have lost their lives to COVID-19
- Vaccination is the mainstay in preventing deaths from COVID-19
- In-hospital treatments that reduce mortality include dexamethasone as demonstrated in the RECOVERY:COVID-19 Dexamethasone trial. Tociluzimab , (and other IL-6 inhibitors) likely improve survival in critically ill patients if given concomitantly with dexamethasone. The jury is out as to whether Remdesivir improves outcomes in the critically ill
- Baricitinib is a Janus kinase (JAK1/2) inhibitor, traditionally used in auto-immune diseases such as Rheumatoid Arthritis. It is known to have anti-cytokine properties and a potential anti-viral mechanism
- Barictinib was studied in combination with Remdesivir in the ACTT-2 study. This demonstrated a reduction in time to recovery compared with patients receiving Remdesivir alone. It was not powered to detect a mortality difference
Design
- COV-BARRIER was a multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 trial
- Randomisation occurred by a computer-generated random sequence to ensure allocation concealment
- Stratification was according to the NIAID-OS (National Institute of Allergy and Infectious Disease Ordinal Scale)

- Patients were included if they had a NIAID-OS of 4 (hospitalised, no supplemental O2 – requiring ongoing medical care), 5 (hospitalised requiring supplemental O2) or 6 (hospitalised, on non-invasive ventilation or HFNO) Table 1 COV-BARRIER NIAID COV-BARRIER NIAID. After October 2020 only patients with NIAID-OS of 5 or 6 were included
- Stratification also occurred for region and use of corticosteroid (low dose dexamethasone allowed)
- Participants, study staff and investigators were blind to the study assignment. The interventions were packaged in identical bottles containing either baricitinib or matched placebo
- An interim analysis was performed by an independent data monitoring committee, which assessed safety, efficacy and futility
- Adverse events were monitored by a blinded safety committee. Baricitinib has been reported to cause venous thromboembolism and predispose to secondary infections, so these events were a particular focus for the safety committee
- The sample size was originally calculated at 400, but during the study increased (before un-masking) to 1400 based on information available from other studies. With 1400 patients the study had 81% power to detect a true treatment effect size of 7·5% for the primary outcome. The final number randomised was 1525
- Registered on clinicaltrials.gov
Setting
- 12 countries, including 101 centres in Asia, Europe, North America and South America. Brazil (22.1%), USA (21%), Mexico (18.4%) and Argentina (13.6%) had the highest proportion of participants
- Patients were enrolled from June 11, 2020, to January 15, 2021, with follow-up complete in March 2021
Population
- Inclusion: Hospitalised patient with laboratory proven SARS-CoV-2 COVID-19 pneumonia and had at least one elevated inflammatory marker > Upper limit of normal (CRP, D-dimer, LDH or ferritin)
- NIAID-OS 4, 5 or 6
- Following release of the results of the ACTT-2 study (October 2020), enrolment was limited to participants with NIAID-OS 5 or 6
- Exclusion:
- Receiving mechanical ventilation with or without other organ support (NIAID-OS 7)
- On immunosuppressants (high dose corticosteroids, anti-IL6, T-cell or B-cell targeted therapies, interferon or other JAK inhibitors)
- Had received convalescent plasma or iv immunoglobulin
- Neutropenic (<1000 cells per μL) or Lymphopenic (<200 cells per μL)
- ALT or AST > 5x normal
- eGFR < 30ml/min/m2 (a half dose, ie 2mg/daily was given if eGFR 30-60ml/min/m2)
- Pregnant
- Thought to have unsurvivable disease
- Baseline characteristics were well-matched between groups
- Baricitinib (n=764) vs. Control group (n=761)
- NIAID-OS 4: 12% vs 13%
- NIAID-OS 5: 64% vs 62%
- NIAID-OS 6: 24% vs 25%
- Concomitant remdesivir: 18% vs 19%
- Concomitant dexamethasone: 92% vs 90%
- Median CRP: 67.5 vs 62·0 mg/L
- Baricitinib (n=764) vs. Control group (n=761)
Intervention
- Baricitinib
- 4mg daily per oral or crushed, dissolved in water and given via NGT for up to 14 days (or until hospital discharge)
- If eGFR 30-60 ml/min/m2, 2mg daily was given instead
Control
- Placebo
Management common to both groups
- Treatment was not protocolised but was at physician discretion at each participating site
Outcome
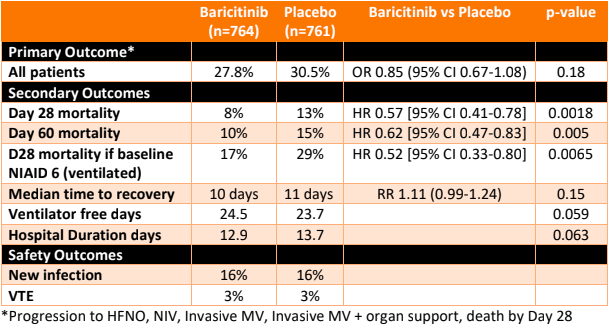
- Primary outcome: There was no significant difference in a composite endpoint of progression in NIAID-OS (score 6,7 or 8) by Day 28 in the baricitinib group vs the control group
- This was an intention to treat analysis in
- Population 1; All randomised participants (n=1518)
- 27.8% vs 30.5% [OR 0.85, 95% CI 0.67-1.08, p=0.18]
- Population 2; The subpopulation needing O2 at baseline & NOT receiving corticosteroids (n=205)
- 28.9% vs 27.1% [OR 1.12, 95% CI 0.58-2.16]
- Population 1; All randomised participants (n=1518)
- This was an intention to treat analysis in
- Secondary outcomes: Baricitinib vs Control
- A significant reduction in All Cause Mortality with Baricitinib vs placebo
- In population 1
- At D28
- 8% vs 13% [HR 0.57, 95% CI 0.41-0.78, p=0.0018]
- NNT 20
- Fragility index 14
- At D60
- 10% vs 15% [HR 0.62, 95% CI 0.47-0.83, p= 0.005]
- At D28
- in population 2
- At D28
- 5% vs 15% [HR 0.31, 95% CI 0.11-0.88, p = 0.030]
- Fragility index 1
- At D60
- 5% vs 18% [HR 0.27, 95% CI 0.1-0.75, p =0.008]
- At D28
- In population 1
- In a subgroup of population 1, D28 mortality:
- NIAD-OS 4: 1% vs 4%, p=0.23
- NIAD-OS 5: 6% vs 9%, p=0.11
- NIAD-OS 6: 17% vs 29%, p=0.0065
- Where the baseline NIAID-OS score was 6, the reduction in D28 mortality was significantly lower (not significant if baseline NIAID-OS was 4 or 5), suggesting in this subgroup, one additional death was prevented per nine baricitinib-treated participants.
- No significant difference in
- Proportion of participants with at least a 1 point improvement in NIAID-OS at Days 4, 7, 10, 14.
- Proportion of participants discharged at Day 4, 7, 10, 14
- Number of ventilator free days/duration of hospitalisation
- A significant reduction in All Cause Mortality with Baricitinib vs placebo
-
- There was no additional benefit detected in the patients who also received remdesivir (n=287)
- 28 day mortality: 9% vs 11%, p=0.6
- The benefits of baricitinib were independent of receipt of corticosteroids (although only around 9% did not receive steroids, so the numbers in this group are small)
- There was no additional benefit detected in the patients who also received remdesivir (n=287)
- Loss to follow-up: 1.5% of participants received no dose of either baricitinib or placebo or were lost to follow-up and were excluded from the safety analyses but were included in the intention-to-treat population
- Safety: 45% receiving baricitinib were reported to have at least one treatment-emergent adverse vs 44% receiving placebo, with serious infections, thromboembolic event and major adverse cardiovascular events occurring with similar frequency between groups
Authors’ Conclusions
- Overall, there was no significant difference in the frequency of disease progression between the baricitinib and control groups
- However, patients receiving baricitinib were less likely to die, with a NNT of 20
- There were similar rates of adverse events between the groups
Strengths
- Allocation concealment, double-blinding, intention to treat analysis, near complete follow-up
- Outcomes are clinically important and patients were tracked out to 60 days which is longer than most other COVID-19 trials and is more representative of the natural timeline of severe disease where patients may still die after a protracted illness
- Independent committee conducted an interim analysis and safety assessment
- The trial was conducted in multiple countries and sites, improving the external validity of the results
- The adjusted sample size likely provides power to detect differences in the key outcomes
Weaknesses
- Given there was no difference in the primary outcome, the key secondary outcomes can not be said to have traditional statistical significance but statistical modelling provided the reported benefits, with nominal p-values assigned
- The benefit of baricitinib in reducing mortality was largely seen in the subgroup of patients who were sicker at baseline. It is difficult to draw firm conclusions from a subgroup analysis but given the overall trend towards benefit, in the patients with higher NIAID-OS at baseline, the results are plausible
- The treatments were not protocolised and there may be heterogeneity across or within institutions, eg criteria for intubation. This could impact on the outcome which looked at improvement in NIAID-OS
- The sample size changed, as did the inclusion criteria, part-way through the trial. However, this adaptation in design was entirely appropriate given the emerging evidence of treatments and the unfolding pandemic
- The trial does not examine costs. In Australia, a 14-day course of baricitinib costs ~ AUD$1400 ($US1000). This is a similar cost to tociluzimab ($800 x 2 doses), which is often used as an alternative agent to baricitinib (although tociluzimab’s use remains controversial). In addition, baricitinib is currently readily available in my practice but tociluzimab is in short supply. The cost, although not excessive, may present a barrier to use in low and middle income nations (although for now, Eli Lilly will donate stock to these countries)
- The ideal dose and duration of baricitinib is not known as there have not been comparative dose seeking trials in this cohort
- It is not known if baricitinib should be used instead of tociluzimab or in conjunction with it. Hopefully, there will be more research to address this question.
- The impact on mortality of patients with baseline NIAID-OS 7 (ie ventilated +/- receiving some other type of organ support) is not clear from this trial, however, further research is planned
- The trial was funded by Eli Lilly pharmaceutical company, who provided research grants to some of the investigators, and some of the investigators served as an advisory board member for Eli Lilly
The Bottom Line
- This trial encourages me to continue using baricitinib in my ICU patients with COVID-19: it looks to be safe and effective
- The overall reduction in mortality at day 28 and day 60 is really exciting AND seems to be of particular benefit in those who are sicker at baseline
External Links
- [Paper] COV_BARRIER Trial
- [further reading] ACTT-2 trial
- [further reading] PulmCrit Blog
- [further reading] rebelem.com:COV-BARRIER
Metadata
Summary author: Celia Bradford @celiabradford
Summary date: 6th September 2021
Peer-review editor: David Slessor
Image by Nick Fewings on Unsplash



