IPHIVAP

Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients?
Bandeshe. J Crit Care 2016; 34:95-102. doi:10.1016/j.jcrc.2016.04.005
Clinical Question
- In adult mechanically ventilated patients, does inhaled heparin compared to inhaled saline or usual care reduce the incidence of ventilator acquired pneumonia (VAP)?
Design
- Feasibility phase 2b study
- Multi-centre
- Randomised, controlled trial
- Double-blind design (treating physicians and patients)
- concealed allocation by off-site computer randomisation
- Permuted block stratification according to study centre and surgical/non-surgical patient status
- Powered at 80% to detect reduction in VAP from expected baseline of 12% to 6%, with statistical significant set at 0.05, if 914 patients recruited
- Trial terminated due to futility after reviewing baseline VAP incidence and a revised power calculation
- Intention to treat analysis
Setting
- Number of centres and location not specified
- “…the majority of patients were recruited from a single center”
- April 2011 to December 2013
Population
- Inclusion: Adult patients over 18 years old; under 24 hours invasive mechanical ventilation; likely to require at least a further 48 hours of mechanical ventilation
- Exclusion: pregnancy; treatment limitations defined; contraindications to heparin; systemic anticoagulation administration; previous enrolment
- Routine heparin for thromboembolism prevention and renal replacement therapy was allowed
- 2103 screened; 214 randomised; none lost to follow-up
- Baseline characteristics
- Mean age: 56 years
- Mean APACHE II score: 18.9
- Mean SOFA score: 6
- Gender: 66% male
- Pneumonia diagnosis on admission: 42%
- Non-surgical admission: 64%
Intervention
- Heparin group
- Nebulised unfractionated heparin 5000 units
- Made up to 2 ml with 0.9% saline
- administered every 6 hours
Control
- Saline group
- 0.9% sodium chloride 2 ml
- Administered every 6 hours
- Usual Care group
- No nebulised heparin or saline
- Allocation to this group was not ‘blinded’ to the clinical staff
Management common to all groups
- Study drug administered regularly until no longer mechanically ventilated for more than 48 hours or discharge from ICU
- If re-intubated for further mechanical ventilation, the study drug was administered according to the patient’s previous allocation
- Humidification and all non-saline/heparin nebulised therapies were allowed
- Pneumonia was treated according to standard recommendations, with at least 5 days antibiotics
Outcome
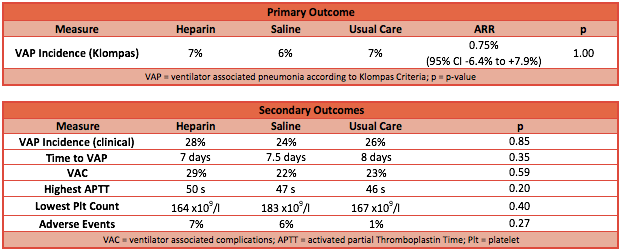
- Primary outcome: No difference in the incidence of VAP
- Clinical diagnosis: heparin group 28% vs saline group 24% vs usual care group 26%; P=0.85
- Klompas criteria: heparin group 7% vs saline group 6% vs usual care group 7%; P=1.0
- Secondary outcome: No significant differences found (heparin vs saline vs usual care)
- Time to VAP: 7 days vs 7.5 days vs 8 days; P=0.35
- Ventilator associated complications: 29% vs 15% vs 18%; P=0.59
- Adverse events: 7% vs 6% vs 1%; P=0.27
Authors’ Conclusions
- Nebulised 5000 units of unfractionated heparin given four times a day is not recommended for the prophylaxis of ventilator associated pneumonia.
Strengths
- Good study methodology so no significant biases likely and probably good accuracy of results (internal validity)
- Appropriate randomisation method and concealment
- Blinded treatment and additional ‘usual care’ group to remove possible influence of nebulised saline
- No patients lost to follow-up
- Objective definition for primary outcome
- Broad inclusion criteria so good external validity and generalisability
Weaknesses
- Under-powered to firmly conclude that heparin has no effect
- Point effect of Absolute Risk Reduction (ARR) for heparin group vs all control group is 0.75% (95% CI -6.41% to 7.91%)
- Authors point out that, if this point effect is accurate and baseline is 6%, a trial with 22,000 patients is required to provide sufficient power for firm conclusion!
- Although probably clinically negligible, this IPHIVAP trial cannot rule out heparin being beneficial (up to ARR 7.9%) or harmful (up to ARI 6.4%)
- With 90% excluded after screening and majority recruited from single centre, some may argue that results are not generalisable
The Bottom Line
- Nebulised heparin is probably ineffective, or the effect is clinically negligible, therefore I shall not be administering this to my patients to prevent ventilator associated pneumonia
External Links
- [article] Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients?
- [further reading] What is the evidence for the use of nebulised heparin in cystic fibrosis
- [further reading] Nebulized anticoagulants for acute lung injury – a systematic review of preclinical and clinical investigations
- [further reading] Rapid and Reproducible Surveillance for Ventilator-Associated Pneumonia by Klompas
Metadata
Summary author: Duncan Chambler
Summary date: 9 February 2017
Peer-review editor: Steve Mathieu



