ITACTIC
Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial
K Baksaas-Aasen et al. @karimbrohi Intensive Care Med 2020. https://doi:10.1007/s00134-020-06266-1
Clinical Question
- In adult trauma patients presenting with signs of haemorrhagic shock does the utilisation of a viscoelastic haemostatic assay (VHA) augmented transfusion strategy compared to a strategy augmented by conventional coagulation tests (CCT) result in an increased number of patients alive and free of massive transfusion at 24 hours?
Background
- The use of VHA guided haemorrhage management is increasing
- TEG and ROTEM are the most commonly used assays, and these allow a rapid, point of care monitoring
- Whole blood is tested and real-time, graphical representation (a temogram) of a patient’s coagulation state is created
- A 2016 Cochrane Review concluded that TEG or ROTEM guided transfusion strategy may reduce need for blood products and improve mortality
- This was with the caveat that a large proportion of the trials included were cardiac surgical, with a low level of evidence.
- The 5th Edition of European Guidelines on the Management of Major Bleeding and Coagulopathy Following Trauma states that CCT and/or VHA can be used for the early and repeated monitoring of haemostasis (Grade 1C) but in the setting of trauma there is little high quality randomised trials which compare the two approaches
Design
- Multi-centre, randomised trial
- Compared outcomes in patients receiving an empiric major haemorrhage protocol (MHP) supplemented by haemostatic therapy guided by either VHA or CCTs
- Randomised in 1:1 ratio, with block randomisation by centre
- Codes generated by independent statistician
- Allocation was by study personnel opening opaque envelope taken in sequence from a stack at each site
- Planned sample size 392 (196 in each arm)
- Based on prior data from centres involved that 28% die or receive MHP at 24 hours and
- An anticipated reduction to 15% (13% relative risk reduction) required 170 patients in each arm (80% power, significance 0.05)
- This was increased to 196 to allow for a drop out rate of 15%
- All data analysed primarily as intention to treat but also as per protocol
- as per protocol excluded those who did not have at least 1 VHA or CCT, did not meet inclusion, died or reached haemostasis within 60 minutes of baseline sampling, or received the wrong test
- Pre-specified subgroups included TBI, prolonged PTr at baseline, on oral anticoagulants and those who received massive transfusion
- Multiple secondary outcomes defined including safety outcomes
- Treating teams unblinded, but research teams collecting data were blinded
- Appropriate consent procedures in place
- Independent data monitoring and trial steering committee
- National ethical approval for all countries and registered with ClinicalTrials.gov
Setting
- 7 major trauma centres in Europe
- Denmark, Netherlands, Norway, Germany, UK
- 1st June 2016 – 30th July 2018
Population
- Inclusion:
- Adult trauma patients
- Clinical signs of bleeding
- Local MHP activation and packed red blood cell (PRBC) transfusion initiated
- Within 3 hr of injury and 1 hr after admission to ED
- Exclusion:
- Nil
- 1308 screened > 479 eligible > 411 randomised
- 203 to CCT group
- 8 did not provide informed consent so 195 in ITT analysis
- 208 to VHA group
- 7 did not provide informed consent so 201 in ITT analysis
- 203 to CCT group
- Demographics, injuries and admission physiology similar (CCT vs VHA)
- Median Age: 43 v 40
- Male: 82% v 73%
- Prior oral anticoagulants: 8% v 6%
- Isolated blunt trauma: 67% v 66%
- Median ISS: 26 v 26
- Severe TBI: 18% v 20%
- Median HR: 105 v 103
- Median Systolic: BP 90 v 95
- Median Lactate: 4.4 v 4.5
- Median fibrinogen: 2.0 v 1.9
- Patients with PTr > 1.2: 32% vs 25%
- Pre-baseline therapy
- Received TXA: 98% v 94%
- Median units PRBC: 2 v 2
- Median units of FFP, Fibrinogen or Platelets: 0 v 0
Intervention
- Use of viscoelastic haemostatic assay (VHA)
- Clear parameters given for blood product replacement for both ROTEM and TEG readings
- VHA tests were performed at point of care and parameters acted upon as they appear, rather than waiting until completion of test
Control
- Use of conventional coagulation tests (CCT)
- Clear parameters for given for blood product replacement
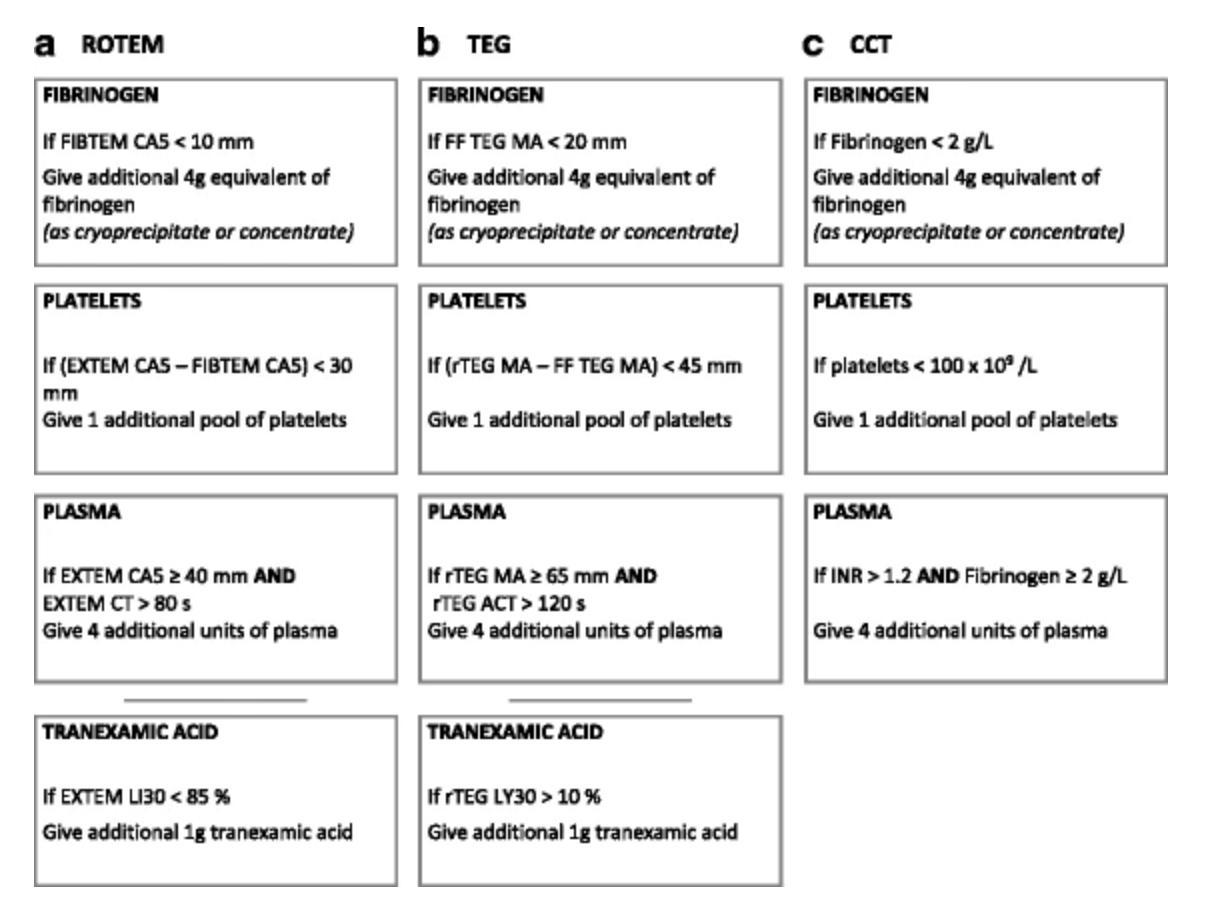
Algorithms used for blood product replacement. Taken from trial protocol (https://doi.org/10.1186/s13063-017-2224-9)
Management common to both groups
- All patients received local hospital MHP
- All received blood products in 1:1:1 ratio of PRBC : platelets : plasma
- VHA or CCT algorithms are to guide additional replacement of fibrinogen, platelets, plasma or TXA
- Limited crystalloid was administered
- Intervention schedule clearly defined as to when blood sampling could occur:
- Resuscitation: ABG at baseline and haemostasis, CCT or VHA after every 4th unit of packed red blood cell until haemostasis
- 6 and 24 hours: VHA and CCT taken along with ABG and haematology and biochemistry at 24 hours
- Haemostasis defined as 1 hr post last transfusion PRBC and clinician satisfaction that haemostasis has been achieved
- Massive transfusion defined as > 10u PRBC
Outcome
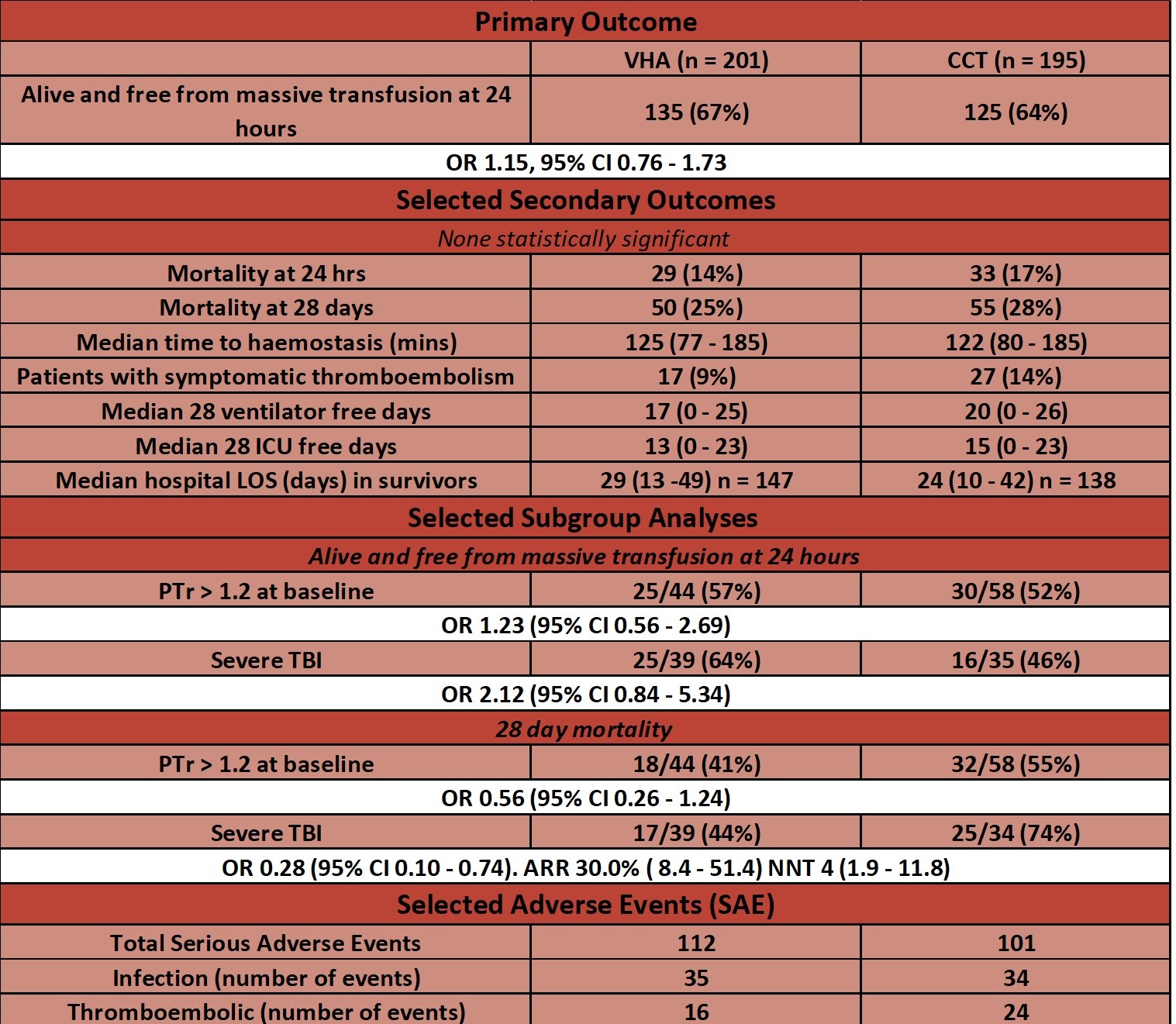
- Primary outcome:
- At 24 hours there was no significant difference between VHA vs. CCT groups in the proportion of patients alive and free of massive transfusion
- 67% vs. 64% OR 1.15 (95% CI 0.76 – 1.73)
- At 24 hours there was no significant difference between VHA vs. CCT groups in the proportion of patients alive and free of massive transfusion
- Secondary outcomes: – no significant difference in any secondary outcome
- Listed in table below
- Selected sub-group analyses
- Severe traumatic brain injury
- 28 day mortality – significantly reduced in VHA group
- 44% vs. 74%
- p = 0.016
- 44% vs. 74%
- 28 day mortality – significantly reduced in VHA group
- Severe traumatic brain injury
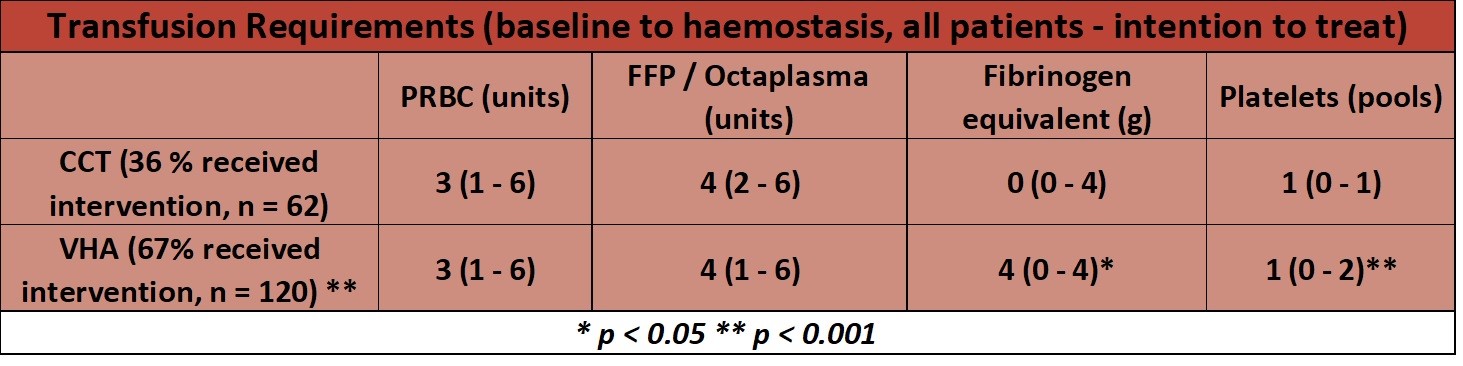
- Blood product usage
- post-hoc analysis
- Timing of Interventions
- post – hoc analysis
- Interventions given a median of 21 minutes faster in VHA vs. CCT group
- 61 minutes (IQR 40 – 85) vs. 80minutes (IQR 60 – 106)
- p < 0.05
- 61 minutes (IQR 40 – 85) vs. 80minutes (IQR 60 – 106)
Authors’ Conclusions
- VHA augmented MHP testing did not improve overall outcomes when compared to CCT augmented MHP
Strengths
- Well Randomised, multi-centre trial
- Good external validity and allows for extrapolation of results to high income countries for trauma patients treated at major trauma centres
- Good randomisation minimises selection bias
- ISS suggests that most patients severely injured with median ISS 26
- Fast time to randomisation (median time from injury to admission 69 minutes)
- Patient focused outcomes reported with sensible safety and adverse event reporting
- In particular VTE prevalence is sensible given potential for increased use of fibrinogen in VHA arm
- Algorithms used for treatment based on prospective, multi centre observational study
- Analysed primarily on intention to treat basis
- As-per-protocol analysis useful given large numbers that have been excluded
- These exclusion criteria for the as per protocol analysis seem sensible
- This is a well done, methodologically sound trial which would be challenging to conduct, given the time critical nature of major trauma patients and other rapidly, evolving competing management priorities and even though “negative” adds a wealth of evidence to traumatic haemorrhage management
Weaknesses
- ISS score suggests patients severely injured however only 28% in the CCT and 26% in VHA group had received MHP at 24 hours
- Low occurrence of trauma induced coagulopathy (TIC) as only 36% in CCT and 67% in VHA group (ITT analysis) received any study intervention (blood products), and ~75% did not have prolonged PTr at baseline
- The patient’s that would benefit from rapid POC testing and blood product replacement are most likely to be those with TIC
- Secondary outcomes and post-hoc analyses not adjusted for multiple comparisons
- In particular this includes intervention timing and blood product usage
- Post-hoc analyses also had up to 9% of records missing in the ITT analysis
- Traumatic haemorrhage is a heterogeneous process
- No data on proportion of patients was significant surgical bleeding was provided i.e. an arterial bleed requiring IR
- It is unlikely that a difference in primary outcome would be detected between the VHA and CCT groups until significant surgical bleeding fixed
- For the per protocol analysis 83 patients (21%) excluded from the ITT analysis
- The authors have been open about this throughout and there was no statistical difference as to the primary outcome for either ITT or as per protocol analysis however:
- 54 excluded for reaching haemostasis in the first hour
- 36 of these were in the VHA group (compared to 18 in CCT group)
- This is highly likely to be chance but a sub group analysis looking at what these patients received would be interesting as it is plausible that maybe some of the treatment provided helped with haemostasis
- 54 excluded for reaching haemostasis in the first hour
- The authors have been open about this throughout and there was no statistical difference as to the primary outcome for either ITT or as per protocol analysis however:
- No data given as to which sites used TEG and which used ROTEM – just stated “balanced use of devices across the study”
- This might not seem significant however ROTEM has values that are available at 5 minutes (CA5) whereas the TEG reading for fibrinogen replacement relies on the maximal amplitude reading (MA) which usually takes longer to appear. One of the purported benefits of VHA is their timeliness to provide results which can be actioned
- Despite being major trauma centres most did not have VHA guided transfusion at the time of study initiation
- Would it be more likely to see benefits with VHA use in centres where VHA use was already widely used, and staff used to using the treatment algorithms?
- As mentioned, this trial provides valid and useful evidence to an area with limited randomised research. However, I feel the use of an augmented protocol in this trial raises some interesting considerations before one can conclude that there is no role for VHA use in traumatic haemorrhage (the authors do not draw this conclusion)
- These are not criticisms per se but more some consideration surrounding how VHA were used in this trial
- These maybe a reflection of pre existing biases but regardless any discussion generated is important
- Would it have been better to compare a stepwise VHA guided algorithm to conventional CCT and empiric treatment approach?
- 1:1:1 replacement as per in this trial may have given products to patients that a VHA may have shown unnecessary
- The lack of a step-wise, sequential VHA algorithm may again result in some products that were given that may not have been required
- i.e. if fibrinogen was replaced as the first step, then this may have been sufficient and thus result in no requirement for FFP and platelets
- Examples of sequential ROTEM algorithms are the Gold Coast University Hospital algorithm (this isn’t open access so the Sir Charles Gairdner Hospital MHP is similar but for all critical bleeding)
- Hypothetically, the administration of products in 1:1:1 ratios in both arms and the lack of a sequential VHA algorithm might mask some of the benefits shown in other VHA trials. Whilst fully acknowledging that this trial provides some of the highest quality evidence on VHA use in trauma, if some of these blood products were not have required, this could lead to greater divergence in blood product replacement between groups
- This potentially may show some benefit of VHA use with regards to patient focused outcomes
- VHA would need to be repeated 10 minutes after any treatment to enable an assessment of response and promptly guide further blood product replacement with the lack of empiric treatment being given
- In this study the chosen point to repeat VHA / CCT was after every 4 units of PRBC given (of which there is no median time provided for how long this period of time was)
- However, the rationale that it is an easy interval to track is logical and using a target such as this enables standardization in both arms. Otherwise there would be an increase in VHA compared to CCT, which no doubt would also be viewed as a flaw
- These are not criticisms per se but more some consideration surrounding how VHA were used in this trial
The Bottom Line
- In adult trauma patients presenting with signs of haemorrhagic shock, this trial did not show benefit of viscoelastic haemostatic assay augmented protocols when compared to conventional coagulation tests augmented protocols
- This is a well done, pragmatic trial set out to try and address an area with limited randomised research however given my reservations above, and some of the trends shown, further studies would be warranted before concluding that VHA has no place in the management of bleeding trauma patients
- Examples of further work could include the use of a stepwise sequential protocol, having trauma induced coagulopathy as an inclusion criteria, and the use in TBI
External Links
[further reading] The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline
[further reading] LITFL: Thromboelastogram (TEG)
Metadata
Summary author: George Walker (@hgmwalker89)
Summary date: 15th October 2020
Peer-review editor: @davidslessor



