Kacmarek
Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial
Kacmarek. Critical Care Medicine; 2016;44(1)32-42, DOI: 10.1097/CCM.0000000000001383
Clinical Question
- In patients with ARDS does open-lung ventilation compared with ARDSnet ventilation improve 60 day mortality?
Design
- Randomised controlled trial
- Web based randomisation
- Stratified according to centre, age and APACHE II score
- Power calculation: 600 patients would need to be randomised to give an 80% power, with a 5% false positive rate, to detect an absolute risk reduction of 12% from a baseline mortality of 45% in the control group
- Trial terminated early due to low recruitment
Setting
- 20 intensive care units, majority in Spain
- Enrolment began September 2007
- Patient follow-up completed August 2013. Unclear when last patient recruited
Population
- Inclusion criteria:
- Adult patients with acute respiratory distress syndrome, defined according to the American-european consensus conference
- PaO2/FiO2 <200mmHg
- Mechanical ventilation for <96 hours
- Recruited into trial within 48 hours of meeting above criteria
- Exclusion criteria:
- Weight <35kg predicted body weight
- BMI >50
- Intubation as a result of an acute exacerbation of chronic pulmonary disease, asthma, cystic fibrosis etc
- Acute brain injury or intracranial pressure >18mmHg
- Immunosuppressed and had received chemotherapy or radiotherapy in previous 2 months
- Severe cardiac disease (NYHA class 3 or 4; acute coronary syndrome or persistent ventricular tachyarrhythmias)
- High risk of mortality from other causes (Age >80, severe neurological damage, cancer patients in terminal stages)
- 200 patients randomised
- For 12-36 hours post-randomisation all patients ventilated according to ARDSnet protocol and then reassessed to ensure met definition for moderate/severe ARDS
-
Comparing intervention vs. control group – no significant differences in baseline characteristics
- Age: 52.2 vs. 53.4
- APACHE II score 18 vs. 17
- Pneumonia: 54% vs. 53%
- PaO2/FiO2 (P/F) ratio: 121 vs. 114
- Tidal volume ml/kg predicted weight (Vt): 7.4 vs. 7.1
Intervention
- Open-lung ventilation (Ventilation strategy using recruitment manoeuvre (RM) followed by decremental PEEP trial), n=99
- First ensured patient apnoeic and haemodynamically stable
- Then performed
- Recruitment manoeuvre
- Using pressure control ventilation
- PEEP initially set at 25cm H2O, pressure control at 15cm H2O, inspiratory time 3 seconds, I:E 1:1, RR 10
- After 5 breaths pressure control increased to 20cm H2O for 5 breaths
- Then PEEP increased to 30cm H20 for final 20 breaths
- Followed by decremental PEEP trial
- Ventilated with volume assist/control, Vt 4-6ml/kg, PEEP 25, ventilatory rate set at level prior to recruitment manoeuvre
- Following 3 minute stabilisation period, dynamic compliance [Vt / (peak pressure – PEEP)] calculated
- PEEP then decreased in steps of 2cm H2O, until PEEP level corresponding with maximum compliance identified
- Recruitment manoeuvre performed again, and PEEP then set at maximum compliance PEEP + 3cm H2O (Maximum 25)
- Recruitment manoeuvre
- Ventilation mode changed to pressure assist/control to set peak inspiratory pressure <30cm H2O, Vt 4-8ml/kg. If Vt <5ml/kg predicted body weight, plateau pressure allowed to exceed 30cm H20.
- PEEP not modified for 24 hours and only when FiO2 decreased to 0.4. PEEP decreased at a maximum rate of 2cm H2O every 8 hours
- If inability to reduce FiO2 to 0.4 at 24 hours post recruitment manoeuvre then above process repeated. Consider using PEEP level of 40cm H2O and peak pressure of up to 60cm H20 for RM
- Maximum of 3 RM allowed per 24 hours
- Mean of 2.57 RM performed per patient
- 10 patients did not have RM performed as too unstable. In 7 patients RM stopped due to hypotension. In 3 of these patients RM continued post fluid bolus.
Control
- Original ARDS-net protocol ventilation
- Ventilator mode: volume cycled assist/control
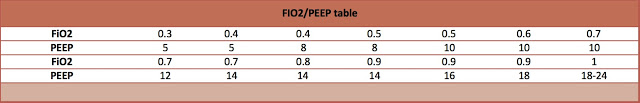
- PEEP and FiO2 adjusted based on ARDSnet table
In both groups
- Vt target 6ml/kg predicted body weight
- Plateau pressure target ≤ 30cm H2O
- Arterial pH target ≥ 7.30 and ≤ 7.45
- All patients assessed daily for spontaneous breathing trial (SBT) based on ARDSnet criteria
- If met criteria had 30-60 minute breathing trial. If passed SBT extubated unless contraindication
- Post-extubation, patients treated with non-invasive ventilation for 24-48 hours until stable or requiring re-intubation if: age >65 years, hypercapnic post extubation (>45mmHg), with an ineffective cough and excessive secretions, with >1 chronic organ failure, upper airway obstruction, APACHE II >12 on day of extubation
- Prone positioned allowed in patients failing to respond to either protocol (4% in intervention group vs. 10% in control group)
Outcome
- Primary outcome: all-mortality death at 60 days – no significant difference
- 29% in intervention group vs. 33% in control group, p=0.18
- Secondary outcomes – comparing intervention vs. control groups
- ICU mortality – no significant difference
- 25% vs. 30%, p=0.53
- Ventilator free days – no significant difference
- 8 vs. 7, p=0.53
- ICU mortality – no significant difference
- Post-hoc analysis
- PaO2/FiO2 ratio at 24 hours post randomisation – significantly improved in open-lung group
- 199mmHg vs. 136mmHg, p<0.0001
- Respiratory variables at day 3
- Plateau pressure: 26.2 vs. 24.5, p<0.05
- PEEP: 14.3 vs. 10.7, p<0.01
- Driving pressure: 11.4 vs. 13.6, p<0.01
- FiO2: 0.4 vs. 0.6, p<0.01
- PaO2/FiO2 ratio at 24 hours post randomisation – significantly improved in open-lung group
- Adverse events
- Major adverse events – no significant difference
- Pneumothorax 6% vs 8%, p=0.78
- Cardiac arrests: 8% vs. 6%, p=0.58
- Minor transient events – no significant difference
- Hypotension: 35% vs. 29%, p=.45
- Desaturation: 34% vs. 22%, p=0.06
- Primary cause of death : comparing intervention to control group
- Progressive respiratory failure: 12% vs. 33%, p=0.11
- Septic shock: 40% vs. 10%, p=0.01
- Multiple organ failure: 16% vs. 33%, p=0.22
- Major adverse events – no significant difference
Authors’ Conclusions
- Open lung ventilation significantly improved oxygenation, lung mechanics and driving pressure, and did not adversely affect duration of ventilatory support, mortality or adverse events
Strengths
- Randomised
- Multi-centre
- Stabilisation period ensured that all patients had moderate/severe ARDS
- Standardised ventilation strategy
Weaknesses
- On day 1 more patients in intervention group vs. control group received neuromuscular blocking agents, and this has been shown to decrease mortality in the ACURASYS Study
- Study under powered and stopped early
- Protocol violations in 13% of intervention group and 12% of control group
The Bottom Line
- In patients with moderate-severe ARDS, the use of open-lung ventilation compared with ARDSnet ventilation improved oxygenation without any significant adverse affects. There was no significant mortality difference, but the study was under powered to detect this. Further, larger trials are required that control for the use of early neuromuscular blockers and the use of proning. Whilst waiting for these trials I will use recruitment manoeuvres with a decremental PEEP trial with the aim of improving oxygenation.
External Links
- [article] Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial
- [further reading] LITFL: Open Lung Approach To Ventilation
- [Trial protocol] Clinicaltrials.gov
- [Podcast from state of the art] Year in review: mechanical ventilation
Metadata
Summary author: Dave Slessor
Summary date: 22nd February 2016
Peer-review editor: Adrian Wong




Is this going to be the future? It intuitively makes sense and the post-hoc outcomes are interesting but sadly this isn’t a game-changing trial for me due to it being under powered: is it a true negative or a false negative?
The open-lung ventilation protocol is so complicated. I have to look it up each time I want to use it. Can you imagine trying to get a whole department to apply it effectively to every severe ARDS patient? Repeat audits have shown that we still can’t do ARDSnet ventilation effectively.
Thanks for the summary Dave and Adrian. Really useful as always.
Great review Dave
The concept of a ventilation strategy using ARDSnet recommendations + recruitment manoeuvre (RM) followed by a decremental PEEP trial makes physiological sense. Using this technique and measuring dynamic compliance has to be a more scientific approach than recruitment with a Mapleson C and APL valve adjusted or ‘guesstimating’ with PEEP levels on a ventilator. With education and repetition, RM and decremental PEEP should become easier to adopt.
However, I share Duncan’s reservations that the study is inadequately powered and we need to improve our overall compliance with standard ARDSnet ventilation first. OSCAR http://www.thebottomline.org.uk/summaries/icm/oscar/ provides a good example of our inability to do the basics consistently well with ARDSnet ventilation.
The benefits of PEEP (at least macroscopically) can be seen here
https://www.youtube.com/watch?v=iuUSDR4ocCY
Pingback: LITFL Review 225 | LITFL: Life in the Fast Lane Medical Blog