DahLIA
Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients with Agitated Delirium. A Randomized Clinical Trial
Reade. JAMA 2016; published on line first March 15th 2016
Clinical Question
- Is Dexmedetomidine effective in reducing the incidence of agitated delirium and days on a ventilator?
Design
- Double-blind, parallel-group, randomised control trial
- Multicenter
- Stratification by site and age (<55 years and >55 years)
- No interim analysis
- Sample size of 96 patients, based on a pilot study, was estimated to provide 80% power to detect a 20 hour difference using a 2-tailed hypothesis at an alpha level of 0.5
Setting
- 15 ICU’s in Australia and New Zealand
- May 2011 – December 2013
Population
- Inclusion: Adult ICU patients who needed to remain mechanically ventilated because their degree of agitation was considered so severe as to make lessening their sedation and extubation unsafe
- This was based on 4 objective measures and patients were eligible for the study if they met any of these in the 4 hours prior to randomisation:
- need for mechanical restraint, antipsychotic or sedative medication
- delirium as defined by Confusion Assessment Method (CAM-ICU)
- Motor Activity Scale (MAAS) score of 5 or greater
- This was based on 4 objective measures and patients were eligible for the study if they met any of these in the 4 hours prior to randomisation:
- Exclusion: pregnant or breast feeding; dementia requiring professional nursing care; had a head injury as the cause of their altered mental status; already receiving dexmedetomidine or clonidine; known contraindication to haloperidol or alpha-agonists
- 21500 patients were screened and 71 patients were randomised
- median age 57 years; 24% women; similar low APACHE II scores; more emergency and cardiovascular patients in the dexmedetomidine group
Intervention
- Dexmedetomidine
- commenced at a rate of 0.5mcg/kg/hr and then titrated to rates between 0 and 1.5mcg/kg/hr to achieve physician-prescribed sedation goals
- clinical-directed option for bolus of 1.0mcg/kg over 20 minutes
- aim was to achieve a Richmond Agitation-Sedation Scale score of 0 or to achieve a physician-prescribed goal
Control
- Placebo
- saline in an identically labelled syringe as the intervention group. Titration and bolus dose option the same as the intervention group
In both groups
- Patients also received opioids and sedatives
- Patients in the placebo group received significantly more antipsychotics meds (65.6% vs 36.8%, 95% CI -51.3,-6.3%, p=0.02), more opioid, and a significantly higher dose of propofol for the 7-days after randomisation.
- The use of midazolam was similar in both groups and there was no statistical difference in the dosage during the 7-days after randomisation
- After 48 hours of study drug infusion, the treating physician could prescribe open-label dexmedetomidine and the study drug infusion would be stopped. More than 7 days of infusion of study drug was considered treatment failure; at that point, the study drug was stopped and open label dexmedetomidine could be commenced
Outcome
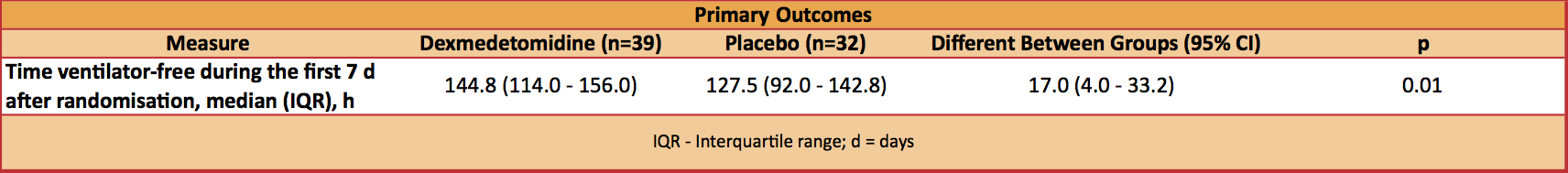
- Primary outcome: Statistically significant increase in median ventilator free hours at 7 days in the dexmedetomidine group
- 144.8 vs. 127.5 hours, P=0.01
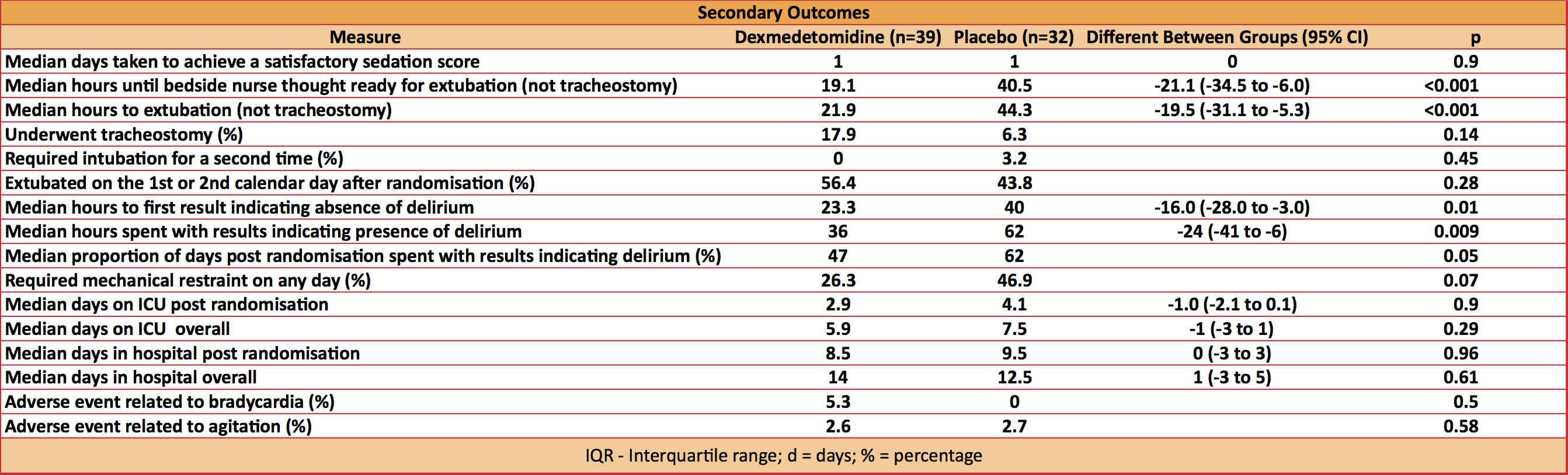
- Secondary outcome: 21 secondary outcomes
- none significantly worse with dexmedetomidine
- 3 favoured dexmedetomidine
- reduced median hours until bedside nurse thought the patient was ready for extubation: 19.1 vs 40.5 hours
- reduced median time to extubation: 21.9 vs 44.3 hours
- accelerated median time to resolution of delirium: 23.3 vs 40.0 hours
- patients in the placebo group received significantly more antipsychotics meds (65.6% vs 36.8%, 95% CI -51.3,-6.3%, p=0.02), more opioid, and a significantly higher dose of propofol for the 7-days after randomisation
- dexmedetomidine was associated with a non-significant decrease in ICU LOS (2.9 vs 4.1 days, -1.0 days, p=0.09)
- No difference in adverse events
Authors’ Conclusions
- Among patients with agitated delirium receiving mechanical ventilation in the Intensive Care Unit, the addition of dexmedetomidine to standard care compared with standard care alone resulted in more ventilator-free hours at 7 days.
Strengths
- RCT
- Multicentre
- Patient-centred primary end point
- Physicians and nurses treating study patients were blinded to group allocation
- Study drugs were prepared by pharmacists or nurses not involved with treating study patients
Weaknesses
- Underpowered. The sponsoring pharmaceutical company declined the option to extend funding beyond the planned completion date. 74 of the planned 96 patients had been randomised at this point
- Details of the choice of sedatives and opioids are supplied for the 24 hours prior to randomisation. However there are no details about the dosage received or what was administered prior to this time.
- A recognition that there was a lack of physician equipoise in this study is considered a strength by the authors. The study accommodated this by permitting open-label dexmedetomidine at 48 hours after randomisation. In fact, a lack of equipoise is arguably an inherent weakness in this study
- Patients in the dexmedetomidine group were ventilated for longer prior to enrolment in the study. Median times were 63 hours vs 43.5 hours
- The MAAS score was used as a component for diagnosing delirium, but further MASS data was then not collected, as part of the criteria for determining resolution of delirium
The Bottom Line
- This underpowered study showed potential benefits of using dexmedetomidine, in addition to standard care, for ventilated patients with agitated delirium, who are otherwise ready for extubation. In particular, there was a modest reduction in time for liberation from the ventilator, more rapid resolution of delirium and sedation and opioid sparing effects. Until there is better evidence and a cost analysis, I will continue to use clonidine as a cheaper alternative to dexmedetomidine
External Links
- [article] Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients with Agitated Delirium. A Randomized Clinical Trial
- [further reading]The Bottom Line summary: MIDEX-PRODEX: Dexmedetomidine vs Midazolam or Propofol for Sedation During Prolonged Mechanical Ventilation: Two Randomized Controlled Trials
- [videocast] Valerie Page explaining CAM-ICU score
Metadata
Summary author: Steve Mathieu
Summary date: date when ready for review
Peer-review editor: Adrian Wong
Poll
[yop_poll id=”1″]




Thanks very much for your comment Paul. Very fair point.
I think the main issues I have with routine use of dexmedetomidine in the specified patient group are:
– this is a relatively small study which is underpowered, accepting it still reaches statistical significance in a number of important clinical outcome measures
– the cost of dexmedetomidine needs to be considered, particularly when there are cheaper alternatives that may have a similar class effect
Dexmedetomidine 100 micrograms/mL, net price 2-mL amp = £15.66; 4-mL vial = £31.32; 10-mL vial = £78.30
Clonidine Tablets, clonidine hydrochloride 25 micrograms, net price 112-tab pack = £10.08 (couldn’t find IV cost)
– Clonidine seems to be effective (lack of level 1 evidence acknowledged). It has a similar effect to dexmedetomidine , although the latter has better selectivity for alpha-2 receptors and therefore probably greater efficacy. However, I wonder how many clinicians have accepted a similar class effect argument for using alternative drugs in the context of other treatments? An example, would be the use of other NMBA’s for refractory hypoxaemia when ACURASYS used cis-atracurium in the intervention group http://www.thebottomline.org.uk/summaries/icm/acurasys/
My continued use of clonidine is more about personal preference rather than any recommendation. If dexmedetomidine was available and cost was not a consideration, I would be more than happy to use dexmedetomidine in patients receiving mechanical ventilation and who have agitated delirium. I do think a cost analysis would be useful though and I would very much like to see a direct comparison between dexmedetomidine and clonidine. Other confounding factors are also increasingly recognised such as avoiding benzodiazepines and non-pharmacological measures for reducing risk of delirium.
Thanks very much
Steve
My biggest issue with this study is it’s incomplete flow diagram.
2.5 years, 15 ICUs, approx 21,500 ICU admissions.
Only 74 patients enrolled.
Assuming the rate of agitated delirium is greater than 0.3%, it would be good to know the characteristics of aggregated delirium patients *not* enrolled and why the other estimated 21,426 admissions were excluded.
Potential serious influence on generalizability.