RECOVERY-RS

An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19
Perkins @perkins_gd medRxiv Pre-print 2021 doi: https://doi.org/10.1101/2021.08.02.21261379
Clinical Question
- In hospitalised patients with COVID-19 requiring >40% FiO2 to achieve SaO2>94%, does the use of continuous positive airway pressure (CPAP) or high flow nasal oxygen (HFNO) compared with conventional O2 therapy (COT) reduce the incidence of tracheal intubation or death within 30 days?
Background
- During the COVID-19 pandemic of 2020-21, critical care resources became scarce, so preserving ventilated beds was prioritised
- Varying guidelines are in place as to the best way to manage patients with progressive moderate hypoxia but there is no firm evidence base to support these guidelines
- Preventing intubation and death are clearly desirable patient-centred outcomes and preserve ventilated beds for those who fail conservative measures
- This trial aimed to assess if CPAP, HFNO or COT reduced the progression to intubation or death
Design
- Open label, adaptive randomised controlled trial
- Sample size initially calculated at 4002 patients with the provision for early stopping if there was a signal towards benefit in early analysis. 3000 patients were required to detect a 5% reduction in the primary outcome from a baseline of 15%, with 90% power and a two-sided 5% significance level. Sample size inflated to 4002 patients due to uncertainties underpinning sample size calculation
- The trial was funded to run for 12 months and at this 12 month point the case numbers of COVID-19 cases diminished considerably so recruitment ceased
- Web-based allocation concealment
- Randomisation stratified by site, sex and age
- The patients were not in ICU, but on respiratory support units
- In centres where all three modalities were available (CPAP, HFNO, COT) allocation was 1:1:1
- In centres where only 2 modalities were available, allocation was 1:1 (CPAP or HFNO vs COT)
- Crossover was defined as changing to a different modality for >6 hours
- Escalation to intubation was at the discretion of the treating teams
- Intention to treat analysis
Setting
- 75 hospitals across the UK
- Recruitment from April 2020 – May 2021
Population
- Inclusion:
- Patients with confirmed or suspected COVID-19
- >18 years
- FiO2>40% and SaO2<94%
- Patient suitable for intubation (no limitations in therapy)
- Exclusion:
- Those needing intubation/ventilation <1 hour after admission
- Pregnant patients
- Those with a contraindication to CPAP or HFNO
- 1277 patients randomised (although 5 were randomised twice, leaving 1272). CPAP in 380, HFNO 417, COT in 475). 8 patients withdrew and 5 patients lost to follow up
- Comparing baseline characteristics
- Well matched at baseline
- 66% were male. Most patients were very fit, fit or well on the frailty score
- At randomisation the mean FiO2 was 60%, SaO2 92.8% and 55.9% had already had awake proning
- Comparing CPAP vs HFNO vs COT group
- Mean age: 57 vs 58 vs 58
- Male: 68% vs vs 65% vs 66%
- White ethnicity: 64% vs 66% vs 66%
- COVID-19 status confirmed: 86% vs 85% vs 86%
- Co-morbidities
- None: 39% vs 34% vs 40%
- Chronic lung disease: 17% vs 13% vs 14%
- Diabetes: 23% vs 24% vs 19%
- Clinical frailty score, p=0.079
- CFS 1 (Very Fit): 19% vs 17% vs 13%
- CFS 2 (Well): 51% vs 47% vs 50%
- CFS 3 (Managing well): 23% vs 26% vs 28%
- CFS 4 (Vulnerable): 3% vs 7% vs 6%
- CFS 5-7 (Mildly – Severely frail): 2% vs 2% vs 2%
- FiO2: 0.62 vs 0.6 vs 0.61
- SpO2: 93% vs 93% vs 93%
- Awake prone positioning: 54.5% vs 58.3% vs 53.1%, p=0.014
Intervention
- CPAP or HFNO
- CPAP
- Mean 9.5cmH20
- Allocation intervention received by 91.6%
- Crossover occurred in 15.3%
- HFNO
- Mean 50.8l/min
- Allocation intervention received by 92.1%
- Crossover occurred in 11.5%
- CPAP
- Breaks from treatment permitted for comfort
Control
- Conventional oxygen therapy
- Allocation intervention received by 98.3%
- Crossover occurred in 23.6%
Management common to both groups
- Patients progressed to intubation at the discretion of their treating clinicians
Outcome
- Primary outcome: Combined end-point of tracheal intubation or death within 30 days
- Significantly reduced in CPAP vs COT group
- CPAP 36.3% (n=377) vs COT 44.4% (n=356) p=0.03
- Unadjusted OR 0.72 (95% CI 0.53-0.96)
- NNT 12 (95% CI 7-105)
- Fragility index 3 patients
- Most of the difference was in intubation rate:
- Intubation within 30 days: 33.4% vs 41.3%, OR 0.71 (0.53-0.96)
- Mortality at 30 days: 16.7% vs 19.2%, OR 0.84 (0.58-1.23)
- No significant difference between HFNO vs COT group
- HFNO 44.4% (n=414) vs COT 45.1% (n=368), p=0.85, unadjusted OR 0.97 (0.73-1.29)
- OR 0.98 for intubation (0.74-1.3), OR for death 0.93 (0.65-1.32)
- Significantly reduced in CPAP vs COT group
- Secondary outcomes:
- For CPAP vs COT
- ICU admission significantly less with CPAP: 54.1% vs 61.5%, OR 0.74 (0.55-0.99)
- Time to intubation significantly longer with CPAP: 2.2 days vs 1.0 day, HR 0.74 (0.58-0.94)
- No significant difference in duration of mechanical ventilation, critical care length of stay or hospital length of stay
- For HFNO vs COT
- No significant difference in ICU admission, time to intubation, duration of mechanical ventilation, critical care length of stay or hospital length of stay
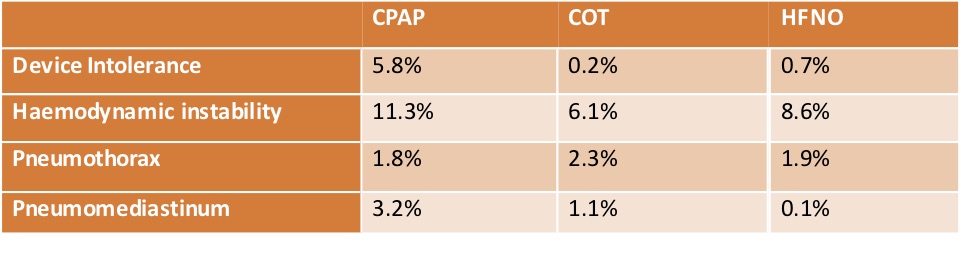
- Safety
- Serious adverse events – significantly increased in CPAP vs COT
- 1.8% vs 0.2%
- Serious adverse events – significantly increased in CPAP vs COT
Authors’ Conclusions
- CPAP, compared with HFNO or COT, reduced the need for intubation or death in hypoxic patients with COVID-19
Strengths
- RCT, allocation concealment, intention to treat analysis, near complete follow-up (98% of data available)
- Primary outcome was a clinically meaningful one and considered resources in the pressured environment of the pandemic
- Multi-centre
Weaknesses
- Unblinded
- Sample size unlikely large enough to detect a mortality difference and did not meet pre-planned sample size
- Crossover was significant but likely does not invalidate the results but may have caused an underestimation in benefit of CPAP
- Difficult to say how generalisable results are to all patients with COVID-19, eg those who aren’t as hypoxic or those not suitable for escalation to intubation
- Low fragility index
- There were no defined criteria for when to intubate patients. In a non-blinded study this may lead to differences between how the groups were treated. Would clinicians be any more or less likely to intubate a patient with a SpO2 of 90% who was on 15l non-rebreathe mask, compared with CPAP with a FiO2 of 0.85?
The Bottom Line
- To me this study would change my practice. In my ICU in Australia, patients who were suitable for intubation, needing high FiO2 (>40%) would be moved into the HDU part of ICU
- I would preference using CPAP (or Bipap) on these patients over HFNO in an effort to reduce the progression to intubation. If the patient is unable to tolerate the device, I would try a variety of mask types to see if there is a preference, eg helmet mask vs face mask vs moon mask
- CPAP, compared with HFNO or COT, reduced the need for intubation or death at 30 days in hypoxic patients with COVID-19. This was predominantly due to the reduction in need for intubation
External Links
- [article] An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19
- [further listening] Critical Care Reviews podcast
Metadata
Summary author: Celia Bradford
Summary date: 16th August 2021
Peer-review editor: @davidslessor
Image by Daniel Büscher from Pixabay




Interesting trial that raises several questions
The biggest question mark on this trial is the control group. Is “conventional oxygen therapy” right up to the point of intubation the standard of care that we currently uphold?
Will be interesting to see the peer reviewed published manuscript in due course
Interesting comment regarding ‘is COT an acceptable way to manage a patient right up until the time of intubation’…. interesting that there was no difference between this and HFNO, as I agree, you would not think this would be the optimal therapy up until this point. Perhaps because resources were so stretched at the time of the study, this was trialled and it proved no worse than HFNO. The RENOVATE trial currently underway in Brazil is comparing HFNO vs NIV in patients with acute respiratory failure. The primary outcome is intubation or death too, so It will be interesting to see if the results of this one support the result of RECOVERY RS trial, although it is not just for COVID patients.
Thanks for your interest/comments
Celia Bradford
Dear Dr Celia Bradford
I’m sure you’ve come across the publication last month in JAMA by Gustavo A. Ospina-Tascón.
https://jamanetwork.com/journals/jama/fullarticle/2786830
It demonstrated that use of HNFO significantly decreased need for mechanical ventilation support compared to COT.
Is it comparable and possibly even contradictory to the some of the results seen in RECOVERY RS?
And would you then also now consider HFNO for patients in acute respiratory failure due to severe COVID 19?
Would love to hear your thoughts and expertise
Thank you so much
Dear Dr Celia Bradford
I’m sure you’ve come across the publication last month in JAMA by Gustavo A. Ospina-Tascón.
https://jamanetwork.com/journals/jama/fullarticle/2786830
It demonstrated that use of HNFO significantly decreased need for mechanical ventilation support compared to COT.
Is it comparable and possibly even contradictory to the results seen in RECOVERY RS?
And would you then also now consider HFNO for patients in acute respiratory failure due to severe COVID 19?
Would love to hear your thoughts and expertise
Thank you so much
Hi!
I’m sure you’ve been made aware of the recent RCT:
https://jamanetwork.com/journals/jama/fullarticle/2786830
Would you have any comments about these 2 trials, the results of the use of HNFO seem different.
May I ask if this has changed your practice?
Thank you