BaSICS

The BaSICS (Balanced Solution versus Saline in Intensive Care Study) Randomized Clinical Trial
(Fluid Type) Zampieri FG; JAMA 2021; doi:10.1001/jama.2021.11684
(Fluid Rate) Zampieri FG; JAMA 2021; doi:10.1001/jama.2021.11444
Clinical Questions
- In adult intensive care patients does the use of a balanced crystalloid compared to 0.9% saline reduce 90-day all-cause mortality?
- In adult intensive care patients needing administration of resuscitation fluids, does the slow infusion compared to a fast infusion of a fluid boluses reduce 90-day all-cause mortality?
Background
- Almost all patients in ICU receive intravenous fluids
- The SPLIT and SMART trials compare Plasma-Lyte and 0.9% saline
- The SPLIT trial, with a median of 2000ml administered in each arm, showed no difference in 90 day AKI
- The SMART trial, with a median of 1000ml administered in each arm, showed a significant greater 30 day occurrence of Major Adverse Kidney Events (a composite of death, new RRT or persistent renal dysfunction)
- The use of rapid fluid boluses, a very common intervention in unwell patients, has been hypothesised to result in unfavourable cardiovascular physiological outcomes, such as reduced arterial elastance
- The FEAST trial showed that in African children with hypotension the use of saline or albumin boluses resulted in an increased risk of 48-hour mortality
- The FENICE study looking at fluid bolus administration in over 2000 ICU patients found that the practice was very variable (with regards to volume, rate, end-points)
Design
- Factorial 2 x 2 randomised study
- Randomised in 1:1:1:1 ratio to each fluid (balanced solution – Plasma-Lyte and 0.9% sodium chloride) and each rate of administration (333ml/hr and 999ml/hr)
- Randomisation via online software in random permutated blocks of 12 patients
- Those involved in patient care were blinded to fluid type but not to the rate of administration
- Fluid supplied in identical 500ml bags labelled with a letter (A – F)
- Data and protocol adherence checked on days 1,2,3 and 7
- Follow up phone call at day 90 to assess vital status and need for RRT
- Power Calculations:
- Planned sample size 11,000
- Based on 35% 90d mortality rate in control (0.9% sodium chloride or rapid infusion)
- 89% power to detect a hazard ratio of 0.9 or less for 90d mortality with an alpha level of 0.05
- Absence of interaction between fluid type and speed was assumed
- Pre-specified secondary, tertiary outcomes and subgroups
- Ideally, prospective informed consent sought but deferred consent allowed
- Appropriate ethical approval gained
Setting
- 75 ICUs in Brazil
- Data collected from 29th May 2017 – 2nd March 2020
Population
- Inclusion:
- Admitted to ICU with a need for fluid resuscitation (defined by 1 sign of hypoperfusion + 1 sign of fluid responsiveness)
- Not expected to be discharged the day after admission
- At least 1 risk factor for AKI (Age > 65 years, hypotension, sepsis, mechanical ventilation, oliguria, raised serum creatinine level, cirrhosis or acute liver failure)
- Exclusion:
- < 18 years
- RRT, or expectation to require RRT, within 6 hours
- Severe electrolyte disturbance (Na+<120 or >160, K+>5.5)
- Death expected within next 24 hours
- Suspected or confirmed brain death
- Receipt of palliative care treatment only
- Prior enrolment
- 11,052 patients randomised
- 486 subsequently refused consent, 46 duplicate randomisations, 25 lost to follow up at 90d
- 5,290 randomised to receive 0.9% sodium chloride
- 0.9% sodium chloride at rapid infusion rate: 2,641
- 0.9% sodium chloride at slower infusion rate: 2,649
- 5,230 randomised to receive balanced solution
- Balanced solution at rapid infusion rate: 2,603
- Balanced solution at slower infusion rate: 2,627
- 5,290 randomised to receive 0.9% sodium chloride
- 486 subsequently refused consent, 46 duplicate randomisations, 25 lost to follow up at 90d
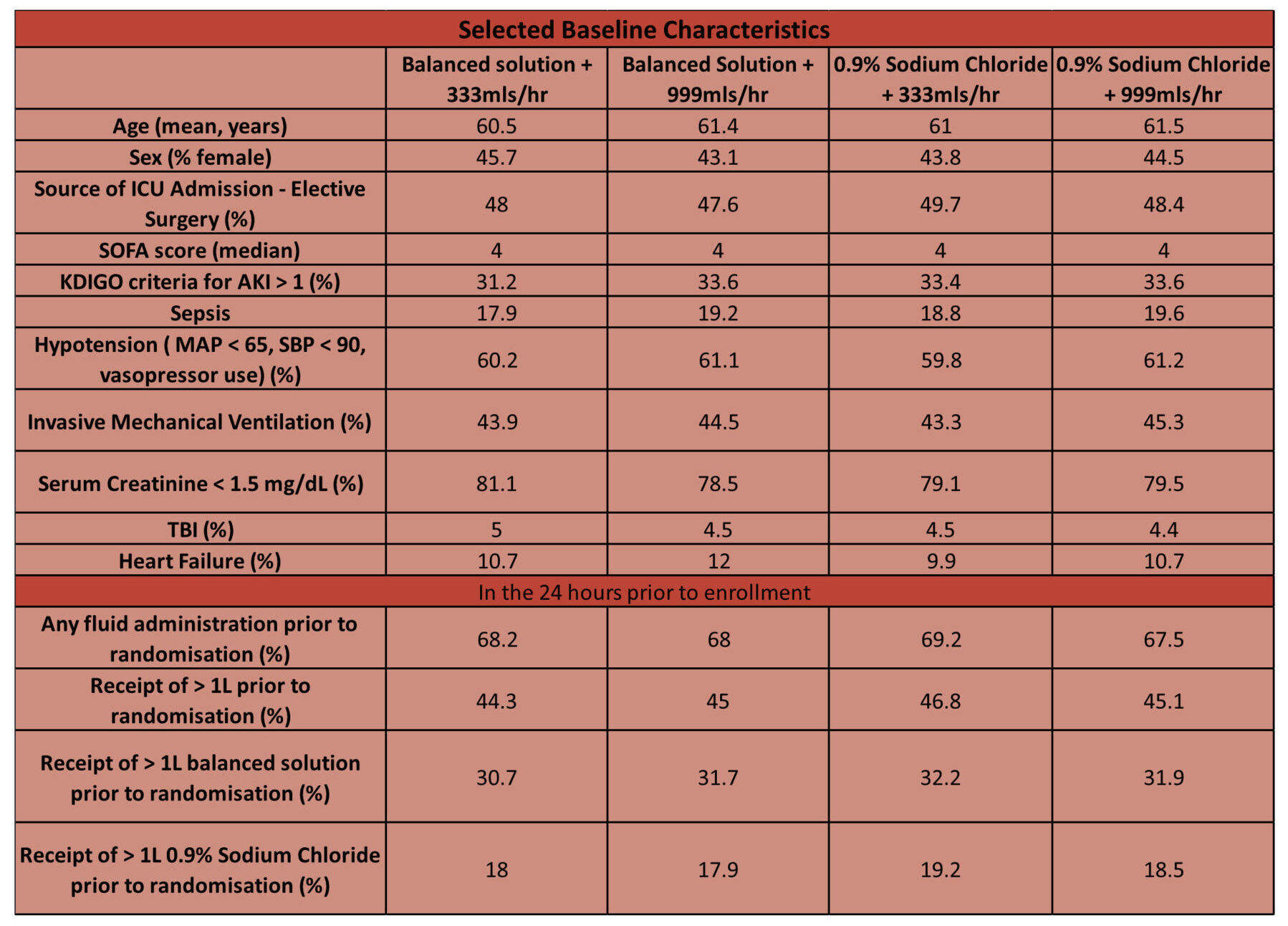
- Comparing baseline characteristics of intervention vs. control group
- Baseline characteristics similar across all groups
Intervention
- Two interventions were assessed:
- The use of a balanced crystalloid (Plasma-Lyte)
- Giving a slower rate infusion (333ml/hr) if fluid boluses were required
Control
- Two controls were assessed:
- The use of 0.9% Sodium Chloride
- Giving a rapid rate infusion (999ml/hr) if fluid boluses were required
Management common to both groups
- Fluid that patient randomised to was used for entire ICU stay (up to 90 days)
- Guidelines provided for when a fluid challenge is required, and when to commence RRT
- Used for resuscitation, maintenance and drug dilution if possible
- If fluid is indicated:
- Maintenance – given study fluid patient randomised
- Fluid bolus – given study fluid at the rate patient randomised
- Other fluid (bicarbonate, albumin, blood etc) – allowed
- If fluid is indicated:
- Volumes and frequency of administration at discretion of treating clinicians
- In some scenarios (e.g. severe hypernatraemia), study fluid not allowed so can use alternate fluid
- Use of study fluid was to resume as soon as contraindication resolved
Outcomes
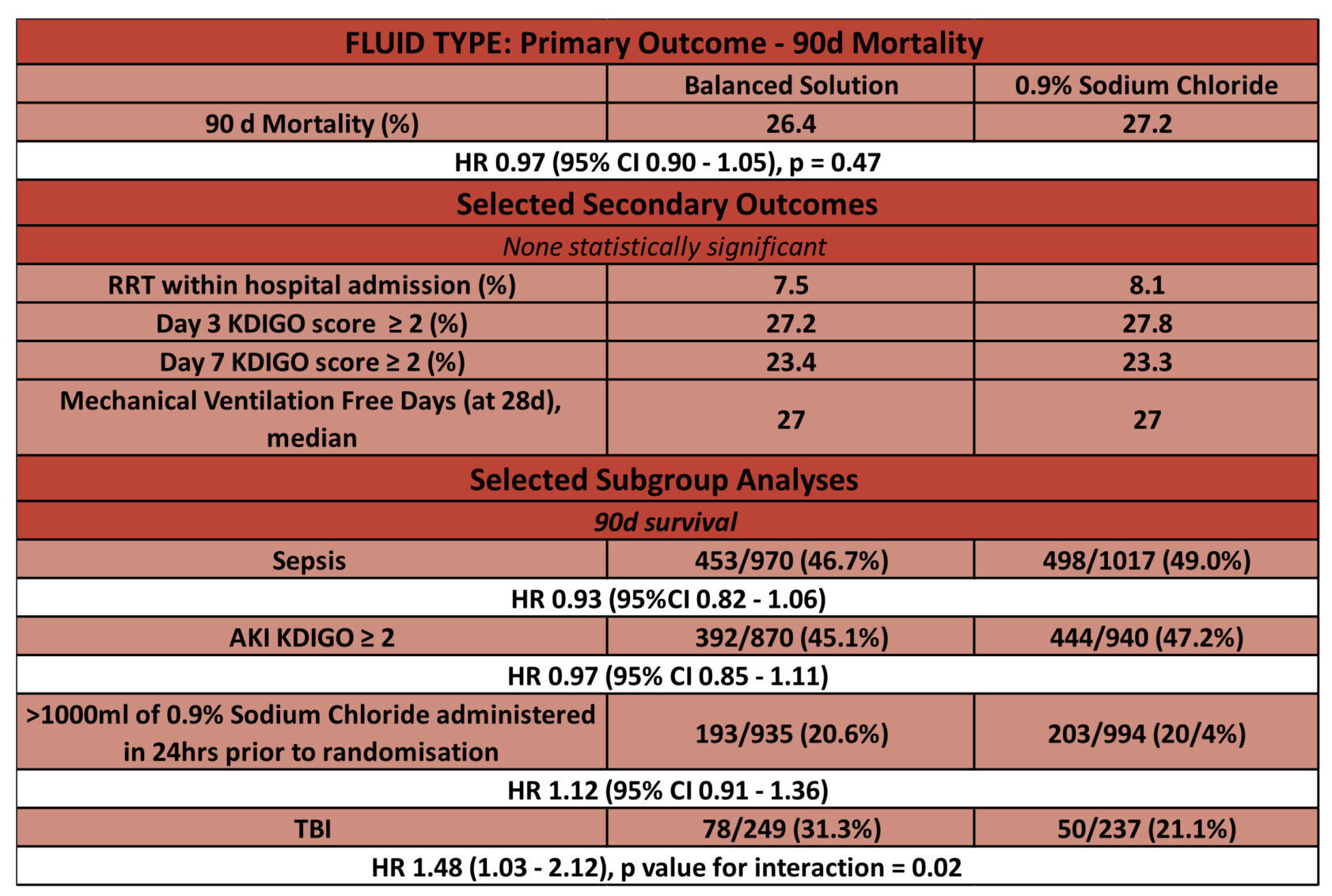
Fluid Type: Sodium Chloride vs. Balanced Solutions
- Primary outcome: No significant difference in 90-day mortality
- 1381 of 5230 patients (26.4%) assigned to a balanced solution died vs 1439 of 5290 patients (27.2%) assigned to saline solution (adjusted HR, 0.97 [95% CI, 0.90-1.05]; P = .47)
- Secondary outcomes: SOFA score at day 7 was significantly different for the balanced solution group
- median difference, 0.27 [95% CI, 0.08-0.45]), mostly due to a higher neurological SOFA score (>2) at day 7 (32.1% vs 26.0% for the saline solution group; odds ratio, 1.40 [95% CI, 1.18-1.66])
- There was no statistical difference in the other 17 secondary outcomes including incidence of AKI with need for RRT within 90 days per 1000 patient days
- Sub-group analyses: statistically significant interaction between presence of traumatic brain injury, fluid type, and 90-day mortality
- 31.3% for the balanced solution group vs 21.1% for the saline solution group [HR, 1.48; 95% CI, 1.03-2.12]
- 26.2% vs 27.5% for patients without a traumatic brain injury
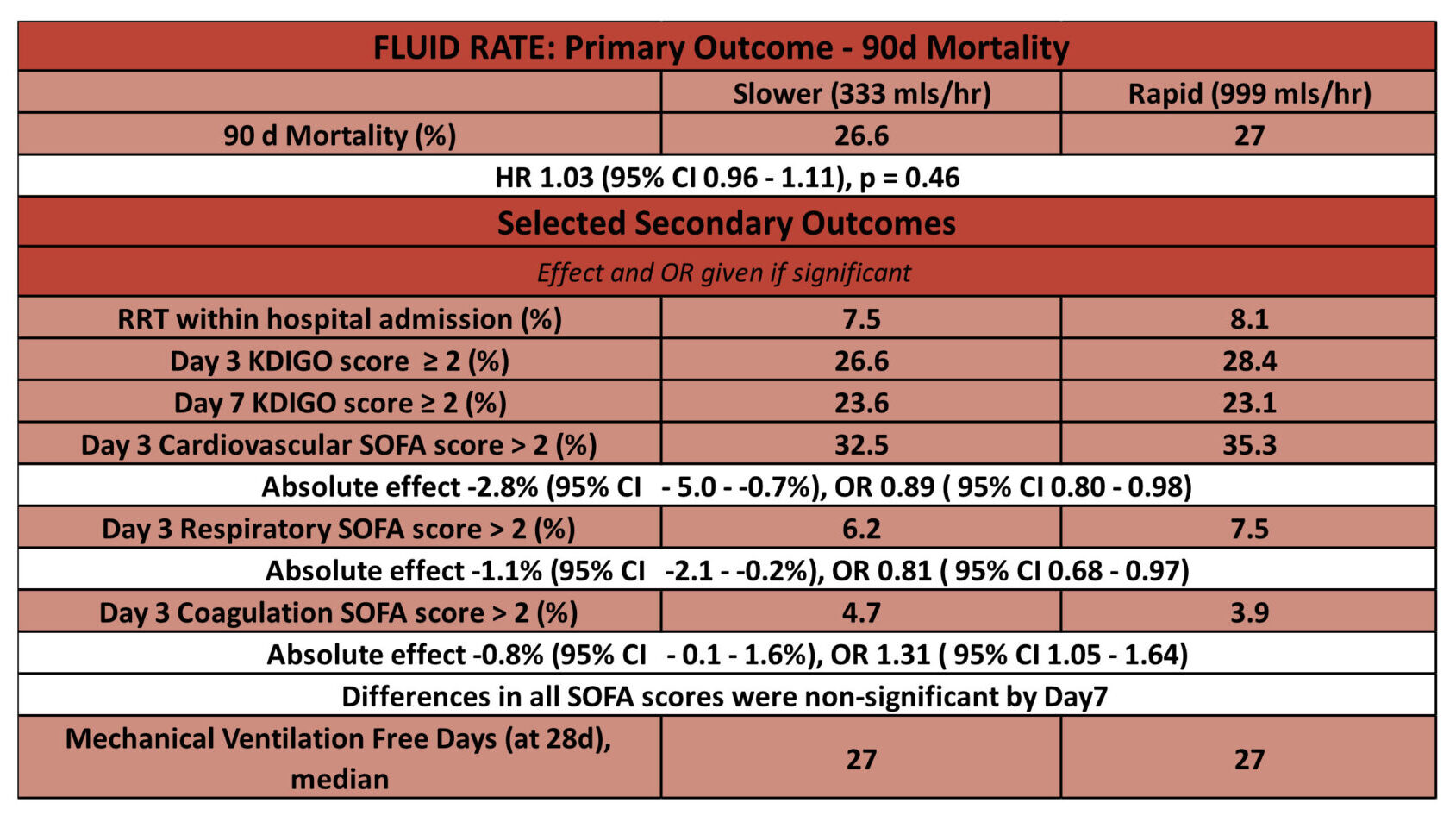
Fluid Rate: Rapid vs. Slow administration of fluid challenges
- Primary outcome: No significant difference in 90-day mortality
- In the slower infusion group, 1406 of 5276 patients (26.6%) had died by day 90 compared with 1414 of 5244 patients (27.0%) in the control group
- HR 1.03 [95% CI 0.96 – 1.11], p = 0.46
- In the slower infusion group, 1406 of 5276 patients (26.6%) had died by day 90 compared with 1414 of 5244 patients (27.0%) in the control group
- Secondary outcomes: differences in Day 3 cardiovascular, respiratory and coagulation SOFA scores significant. By Day 7 no differences in SOFA scores significant.
- Day 3 cardiovascular SOFA scores lower in slower group, 32.5 vs. 35.3
- OR 0.89 [95% CI 0.80 – 0.98]
- Day 3 respiratory SOFA score lower in slower group, 6.2 vs. 7.5
- OR 0.81 [95% CI 0.68 – 0.97]
- Day 3 coagulation SOFA score higher in slower group, 4.7 vs. 3.9
- OR 1.31 [95% CI 1.05 – 1.64]
- Day 3 cardiovascular SOFA scores lower in slower group, 32.5 vs. 35.3
Authors’ Conclusions
- Among patients in intensive care:
- The use of a balanced crystalloid compared to 0.9% sodium chloride did not reduce 90-day mortality
- The use of slower infusion rates when a fluid bolus is required compared to a faster rate of infusion did not reduce 90-day mortality
Strengths
- An extremely large, multi-centre trial designed to answer an important clinical question given the frequency of fluid use in ICU
- Excellent internal validity
- Good randomisation strategy. This strategy was tested at end when principal investigators were asked if they could guess which fluid each letter corresponded too
- 6/66 who replied, correctly guessed the association between all letters and fluid type (this was compatible with hypothesis that the answers guess were random)
- Appropriate blinding for fluid type (not blinded for rate)
- Minimal loss to follow-up: 0.2% (25/11052)
- Balanced baseline characteristics
- Pre-printed statistical analysis plan
- Good randomisation strategy. This strategy was tested at end when principal investigators were asked if they could guess which fluid each letter corresponded too
- Sensible secondary outcomes and pre-specified subgroups
- The authors rightly identify these being as hypothesis generating only
- To complete, process and write up such a large and methodologically rigorous RCT whilst in the midst of Brazil’s COVID-19 situation is impressive
Weaknesses
- Not analysed as intention to treat
- Given that majority (~87%) of those who did not receive treatment as randomised was due to withdrawal of consent, this is unlikely to be clinically relevant
- Mortality lower than anticipated in power calculations (~27% vs. 35%)
- May reduce power of study to detect important differences
- Mostly lower acuity ICU patients (median APACHE II score 12 and SOFA 4) with nearly 50% elective surgical and 40% of patients not hypotensive
- Median volumes of trial fluid administered were low (mean <1 L/day) making a difference in outcomes potentially less likely to be detected
- 24 hours study fluid: 1.5L in each group
- 72 hours total study fluid: 2.9L in each group
- 72 hours total fluid (study and non study fluid): 4.1L (SD 2.9L)
- The administration of non-study fluids could confound results:
- 68% of all patients received fluid prior to randomisation (~32% in the 0.9% sodium chloride received >1L balanced fluid and ~18% in the balanced group received >1L 0.9% sodium chloride).
- Following randomisation ~30% of the total fluid received by day 3 was non-study crystalloid (eTable 2, Supplement 3)
- Additionally, dextrose, albumin and synthetic colloids were also transfused
- Chloride load hypothesised to be reason for increased mortality and organ failure. Whilst mean serum chloride levels were higher in 0.9% sodium chloride group at 48 hour (108 mEq/L vs. ~105.5 mEq/L), this was only 2 mEq/L above the upper limit of normal range which is not a meaningful clinical important difference
- The slower infusion rate was defined arbitrarily at 333 mL/hr. The authors provide explanation that as this is less than 25% percentile in the Fluid Challenges in Intensive Care (FENICE) cohort study (500 mL/h) it still represented a value that was considered as a bolus (and not maintenance) by clinicians
- No data collected on reason for administration of fluid bolus or immediate haemodynamic effects
The Bottom Line
- On the basis of this trial, the use of 0.9% sodium chloride in ICU appears safe
- Given some of the limitations above, and conflicting data from other trials, I will continue to use balanced crystalloid for those patients who are acutely unwell with an unplanned ICU admission
- Although the secondary outcomes are hypothesis generating, it would seem reasonable to use 0.9% saline in TBI patients
- The need for (rapidly administered) fluid boluses for intensive care patients should be evaluated on an individual patient basis, rather than be a reflexive action given lack of benefit and potential harm shown
- The PLUS trial will continue to inform practice
External Links
- [Paper] Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients. The BaSICS Randomized Clinical Trial
- [Paper] Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients. The BaSICS Randomized Clinical Trial
- [Editorial] Does Crystalloid Composition or Rate of Fluid Administration Make a Difference When Resuscitating Patients in the ICU?
- [Further reading] Study Protocol
- [Further reading] Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA)
- [Video presentation] Critical Care Reviews Trials Results Livestream
- [Further reading] The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 11th August 2021
Peer-review editor: Steve Mathieu @stevemathieu75
Picture by: iStock



