RESCUEicp

Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension
Hutchinson. Published on line 7th Sept; NEJM 2016; DOI: 10.1056/NEJMoa1605215
Clinical Question
- In patients with a traumatic brain injury (TBI) and refractory intracranial hypertension, does decompressive craniectomy, result in more favourable mortality and neurological outcomes at 6 months, compared with barbiturate coma and continued medical management?
Design
- International, multicenter randomised controlled trial
- Parallel group superiority
- 1:1 randomiation with the use of permuted blocks of random sizes and with stratification according to trial sizes
- Modified (excluded patients lost to follow up or withdrew consent) intention to treat analysis
- Sample size of 400 patients was calculated to detect a 15% difference in favourable outcome rate between the two groups. A power of 80% allowed for a loss of follow-up of up to 15%
- The primary outcome measure was analysed with an ordinal analysis method based on the proportional odds model
Setting
- 52 centres in 20 countries
- All trial sites were hospitals that provide acute neurosciences care for patients with severe TBI and had access to 24 hour neurosurgical service
- 71% of patients were recruited in the UK
- Jan 2004 – March 2014
Population
- Inclusion: patients with a TBI with an abnormal brain CT and raised intracranial pressure of >25mmHg for 1-12 hours, despite stage 1 and 2 measures; aged between 10 and 65 years
- Exclusion: patients with bilateral fixed and dilated pupils, bleeding diathesis, or an injury that was considered to be unsurvivable
- 2008 patients were assessed for eligibility; 408 randomised; data available for 389 patients at 6 months and 373 patients at 12 months
- Baseline characteristics of the groups were similar except that fewer patients in the craniectomy group had a history of drug or alcohol abuse. Characteristics of the craniectomy group versus the medical group were as follows:
- mean age: 32 vs. 35 years
- Male preponderance 82% vs. 80%
- GCS motor score of 1-2 at first hospital: 53% vs. 50%
- Extracranial injury: 37% vs. 42%
- Injury classification based on CT similar with diffuse injury accounting for over 2/3rd
Intervention
- Decompressive craniectomy
- Continued stage 1 and 2 treatments plus decompressive craniectomy
- Either large unilateral fronto-temporoparietal craniectomy (hemicraniectomy) for patients with unilateral hemispheric swelling or bifrontal craniectomy for patients with diffuse brain swelling
Control
- Medical Group
- Continued stage 1 and 2 treatments plus barbiturates
In both groups
Stage 1 and stage 2 treatments were provided prior to stage 3 therapy (intervention or control group). Stage 3 therapy was commenced if the intracranial pressure > 25mmHg for 1-2 hours after initiation of stage 2 therapy
Stage 1 therapy:
- initial treatment measures: head elevation; ventilation; sedation; analgesia; paralysis (optional)
- monitoring: central venous pressure; arterial blood pressure; intracranial pressure
Stage 2 therapy:
- commenced if intracranial pressure remained > 25mmHg after stage 1 therapy
- included: continuing stage 1 treatments and optional treatments. These included:
- ventriculostomy; inotropes; mannitol; hypertonic saline; loop diuretics; hypothermia
- barbiturares were NOT permitted
Patients assigned to receive medical treatment alone could undergo a decompressive craniectomy later if their condition deteriorated further (37.2%). Similarly those patients assigned to undergo a decompressive craniectomy were permitted to receive a barbituarate infusion for the same indication (9.4%)
Outcome
- Primary outcome: Extended Glasgow Outcome Scale (GOS-E) at 6 months after randomisation in the craniectomy vs. medical group was as follows:
- Death: 26.9% vs. 48.9%
- Vegetative state (VS): 8.5% vs. 2.1%
- Lower severe disability (LSD): 21.9% vs. 14.4%
- Upper severe disability (USD): 15.4% vs. 8%
- Lower moderate disability: 10% vs. 10.1%
- Upper moderate disability: 13.4% vs. 9.6%
- Lower or upper good recovery: 2.5% vs. 3.2%
- Upper good recovery: 1.5% vs. 3.7%
- If GOS-E is grouped into 3 categories – Dead, VS/LSD and USD or better
- craniectomy group: 26.9%, 30.3%, 42.8%
- medical group: 48.9%, 16.5%, 34.6%
- Secondary outcome:
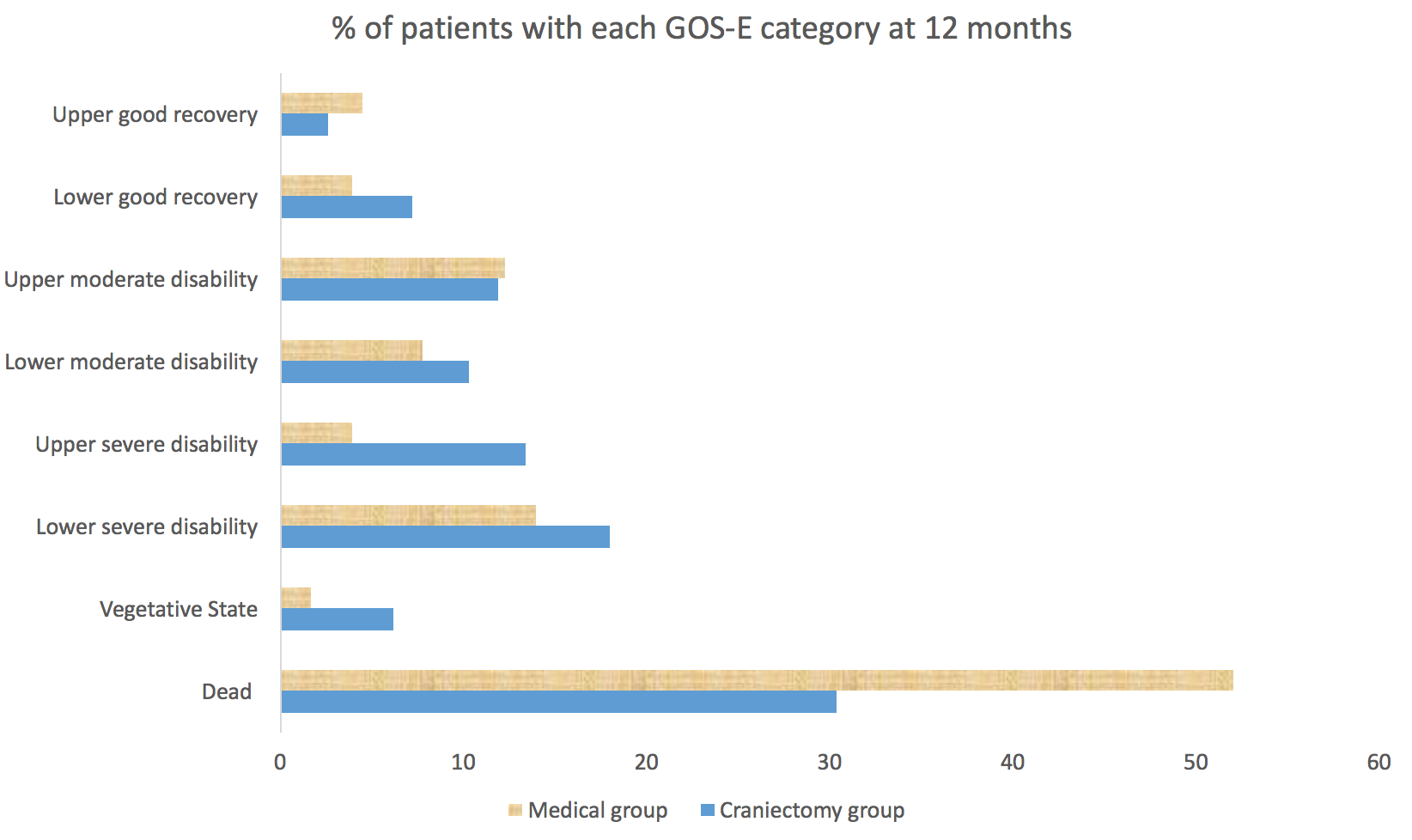
- GOS-E at 12 months after randomisation in the craniectomy vs. medical group were as follows
- death was lower in the craniectomy group: 30.4% vs. 52%
- vegetative state (VS) was higher in the craniectomy group: 6.2% vs. 1.7%
- Lower severe disability (LSD): 18% vs. 14%
- Upper severe disability (USD): 13.4% vs. 3.9%
- Lower moderate disability: 10.3% vs. 7.8%
- Upper moderate disability: 11.9% vs. 12.3%
- Lower or upper good recovery: 7.2% vs. 3.9%
- Upper good recovery: 2.6% vs. 4.5%
- If GOS-E grouped into 3 categories – Dead, VS/LSD and USD or better
- craniectomy group: 30.4%, 24.2%, 45.4%
- medical group: 52%, 15.7%, 32.4%
- Overall it is estimated that for every 100 patients treated with decompressive craniectomy rather than medical intent, there were 22 more survivors; of these 22 patients, 6 were in a vegetative state (27%), 8 were categorised as having lower severe disability (36%), and 8 were categorized as having upper severe disability or better (36%)
- GOS-E at 12 months after randomisation in the craniectomy vs. medical group were as follows
-
- GCS at discharge from the neurosciences hospital
- 18.9% in the craniectomy group vs. 12.2% in the medical group had GCS of 8 or less
- Assessment of intracranial pressure control: favoured the craniectomy group
- Time to death or discharge from ICU: no statistical difference in the median values. The median lengths of stay in ICU are 15 days in the craniectomy group and 20.8 days in the medical group
- GCS at discharge from the neurosciences hospital
- % of patients with at least one reported complication or adverse event: statistically significant increase in the craniectomy group 15.3% vs. 9.2%
Authors’ Conclusions
- At 6 months, decompressive craniectomy in patients with TBI and refractory intracranial hypertension resulted in lower mortality and higher rates of vegetative state, lower severe disability, and upper severe disability than medical care
Strengths
- All trial sites were hospitals that had immediate access to 24 hour neurosurgical services
- Exploratory analyses examining the effect of covariate adjustment (age, GCS, motor score, pupillary reactivity, and the Marshall grade of the CT brain pre-randomisation) were pre-specified
- Similar numbers of patients in the two groups received stage 1 and stage 2 treatments
- Timing for initiation of stage 3 treatment was similar in both groups
- Agreement for participation in the study was obtained from the next of kin preemptively on admission so that initiation of stage 3 therapy was not delayed
- Randomisation code was not released until the patient had reached stage 3 of the protocol
- Extended Glasgow Outcome Scale (GOS-E) is a well validated global outcome measure to assess functional independence, work, social and leisure activities, and personal relationships
- Explanation provided for the reason that GOS-E results were reported descriptively at 6 months. The proportional-odds assumption was rejected
Weaknesses
- Slow recruitment over 10 years with 50% of centres only recruiting 3 or fewer patients
- Therapeutic hypothermia (optional stage 2 treatment in this study) may have a deleterious effect on neurological outcome after TBI based on Eurotherm3235. The study was commenced before Eurotherm3235 was published. Whilst therefore not a criticism of the study design, this may have affected overall GOS-E
- Clinical teams who cared for the patients were aware of trial-group assignments
- A large proportion of patients in the medical group underwent decompressive craniectomy (37%); this situation may have diluted the observed treatment effect
- 10 patients were excluded from all analyses owing to withdrawal of consent
or to a lack of valid consent, and 7 more patients in the medical group were lost to primary follow-up - Long term outcomes of survivors with severe disability would be useful. This follow up data may be planned for future?
The Bottom Line
- Decompressive craniectomy in patients with TBI and persistently raised intracranial pressure, after stage 1 and 2 management, was associated with lower mortality than medical management. However, more survivors in the surgical group than in the medical group were dependent on others. With data now available from DECRA and RESCUEicp, there is likely to be concern that life saving surgery may not predictably result in sufficiently good functional survival. Further investigations, exploring patient selection, longer term recovery and quality of life may be indicated
External Links
- [article] Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension
- [editorial] Intracranial Pressure Rescued by Decompressive Surgery after Traumatic Brain Injury
- [further reading] Decompressive Craniectomy in Diffuse Traumatic Brain Injury
- [further reading] Decompressive Surgery Cuts Death in Traumatic Brain Injury
- [further reading] LITFL: RESCUEicp and the Eye of the Beholder
Metadata
Summary author: Steve Mathieu
Summary date: 8th September 2016
Peer-review editor: Duncan Chambler & Dave Slessor





https://twitter.com/NEJM/status/773638321004216320
No difference in traditional dichotomisations of GOSE 27% in each arm- so you can compare with other trials. Surgical group less deaths and more dependent and severely dependent survivors without any real increase in true independent survivors = huge increase in societal costs. Greater mortality in medical group with no less good outcome = potential for increased organ donation and huge societal benefits and reduced costs – discuss.
You also need to factor in the 20% incidence of complications including death of subsequent cranioplasty that the decompressed patinets will need http://www.ncbi.nlm.nih.gov/pubmed/23875882
I think the answers are not there (yet).
1.why do people favouring decompressive craniectomy always change the classical dichotomisation to make their point. If you take the normal favourable outcomes you have a 27.4 vs 26.6% in favour or equal for medical treatment. actually the same thing happened in the destiny, Hamlet and a third study (I forgot the name) about decra in the case of malignant MCA syndrome.
2. information is lacking of the huge group (37.2%) who had their decra in the medical group. We know they figure in the medical arm of the study, but we don’t have any info about their GOS-E outcome and parameters and timing of the procedure.
3.I’m convinced that with the info of this last group (medical group with decra) could give us some real practical info. The difficult question is which of the classical 3° tier treatments we should use first. We know from the eurotherm and decra that hypothermia and decra respectivily shouldn’t have a place as a second tier treatments. But together with barbiturate coma they remain our last resort weapons in intractable intra cranial hypertension. Actually the Rescue ICP study could help us with this because the medical group is like a barbiturate group, the surgical only decompressive craniectomy and the 37.2% having a decra while in barbiturate coma figure as a combined group. This last group is a situation we see easily in our practice. For this analysis we need to know the GOS-E scores of this last group.
Pingback: Papers of October | The Resus Room
Not only did 37% of patients in the medical group undergo a DC, but another 15% had a craniotomy for hematoma evacuation prior to randomization. I’d be interested to know how many of the patients who had a hematoma evacuation crossed over into the DC group. If none, you could argue that over 50% of patients in the medical group actually underwent a neurosurgical procedure. I’m surprised they found any difference at all in mortality and I’m not surprised there were no differences found in the other end points. It would have been interesting if an as-treated analysis was performed. However, clinical research is very difficult and these authors did a great job of building on prior research and moving us forward. This paper shows us we must communicate with our families and loved ones because the possible outcomes of DC range from very bad to very good and we don’t know who will end up where, YET….
Gary.. thanks for visiting TBL and for taking the time to comment. Your points are very valid. This trial was always going to struggle to prove the hypothesis one way or the other, but it’s probably the best evidence so far. The art is knowing how to apply that to our individual patients.
Pingback: 2016 Review. St.Emlyn's - St.Emlyn's
Pingback: Top 10+1 trauma papers 2016. St.Emlyn's - St.Emlyn's
Pingback: RescueICP and Decompressive Craniectomy by Dash Gantner and Jamie Cooper - INTENSIVE