TELSTAR

Treating Rhythmic and Periodic EEG Patterns in Comatose Survivors of Cardiac Arrest
Ruijter BJ, NEJM February 24 2022;386:724-34. DOI:10.1056/NEJMoa2115998
Clinical Question
- In comatose survivors of cardiac arrest does the treatment of rhythmic and periodic electroencephalographic (EEG) patterns improve neurological outcome compared with standard care?
Background
- Cardiac arrest, occurring both in and out-of-hospital, is associated with high mortality and morbidity
- Even with the absence of clinically apparent seizures, abnormal EEG findings such as both rhythmic and periodic discharges, occur in 10-35% of these patients
- These abnormal EEG findings are a poor prognostic sign and associated with worse outcomes
- There is variation in treatment practices amongst neurologists and it is not known whether pharmacological suppression of this abnormal EEG activity improves outcome
Design
- 1:1 ratio
- Permuted blocks of variable size
- Multi-centre, open-label treatment
- Blinded outcome assessors
- Written informed consent from next of kin
- An independent safety monitoring board analysed the safety and efficacy of the study at key points throughout the trial to advise if it could continue
- Power calculations required 84 patients in each arm to detect superiority
- This was based on a 7% reduction in poor outcome (from 99 – 92%) with anti-seizure treatment, an alpha of 5% and a power of 80%
Setting
- 11 ICUs in Belgium and The Netherlands
- May 1 2014 – January 24 2021, with 3-month follow-up
Population
- Inclusion:
- >18 years
- Comatose survivors of cardiac arrest (in OR out-of-hospital)
- ROSC
- Had continuous EEG monitoring initiated <24 hours after the arrest
- Minimum duration of rhythmic or periodic EEG pattern was for >30 consecutive minutes or lasting >5 minutes at least twice in a 60-minute period
- Exclusion:
- Pre-hospital GOS <3
- Other cause of status epilepticus or progressive brain illness (neurodegenerative disease or brain tumour)
- Life-sustaining therapy not deemed appropriate
- Consent not possible
- 354 patients were found to have rhythmic or periodic EEG pattern.
- 175 were excluded and 179 included for randomisation
- 88 allocated to the intervention group and 84 to the control group
- Comparing baseline characteristics of intervention vs. control group
- Groups were generally similar at baseline except rates of initial shockable rhythm
- Median age: 65
- Male: 69%
- 95% were out of hospital arrest, 5% in hospital
- Presumed to be a cardiac cause of the arrest: 80% v 77%
- Time to commencement of BLS: 5 vs 5 minutes
- Time to ROSC: 16 vs 19 minutes
- Higher rates of shockable rhythm as first monitored rhythm in control group: 60% vs 72%
- All patients received targeted temperature management:
- 24% vs 24% targeted at 33C
- 76% vs 76% targeted 33-36C
- Myoclonus at randomisation: 61% vs 64%
- EEG patterns at randomisation:
- Generalised periodic discharges (0.5 – 2.5Hz): 77% vs 81%
- Electrographic seizures (>2.5Hz): 10 vs 10%
- Suppressed background continuity: 15% vs 17%
- Groups were generally similar at baseline except rates of initial shockable rhythm
Intervention
- 3-staged antiseizure treatment: titrated to EEG
- Step 1: antiepileptic drug + benzodiazepine
- Phenytoin load (15-20 mg/kg), then regular phenytoin titrated to serum level + benzodiazepine bolus + infusion. If phenytoin contraindicated, levetiracetam or valproate were used for step 1
- Step 2: anaesthetic agent + second antiepileptic drug
- Propofol infusion (8mg/kg/hr) + levetiracetam (1500mg load + 1g bd maintenance) OR valproate (10-20 mg/kg load + 7.5mg/kg bd maintenance)
- Step 3: second anaesthetic agent
- Thiopental: first six hours 12.5 mg/kg/hr iv, next six hours 5 mg/kg/hr iv
- Step 1: antiepileptic drug + benzodiazepine
- The goal of the antiseizure therapy was to suppress all rhythmic and periodic EEG activity for at least 48 consecutive hours (>90% suppression)
- Treatment started within 3 hours of detection of rhythmic and periodic EEG patterns
- Treatment guided by neurologist or clinical neurophysiologist who adjusted medications in consultation with ICU physician
- EEG recordings checked every 3 hours
- If the abnormal EEG patterns were observed beyond 48 hours, the treatments could be continued beyond this point if the physician preferred
Control
- Physician preference: sedative agents could be prescribed if there was ventilator dyssynchrony or myoclonus
- Use of antiepileptic agents discouraged although 8 patients in this group did receive at least 1 antiepileptic agent
Management common to both groups
- At the discretion of the treating team
- Targeted temperature management similar in both groups
- Limitations and withdrawal of active therapies were based on Dutch Guidelines and were not determined by the presence or absence of abnormal EEG patterns
Outcome
- Primary outcome: Neurologic outcome according to the Cerebral Performance Category scale at 3 months dichotomized to good outcome (CPC 1-2) or poor outcome (CPC 3-5) [comparing intervention vs control]:
- No difference in CPC 3-5: 90% vs 92% (p=0.68)
- Risk difference 2% (95% confidence interval -7 to 11)
- No difference in CPC 3-5: 90% vs 92% (p=0.68)
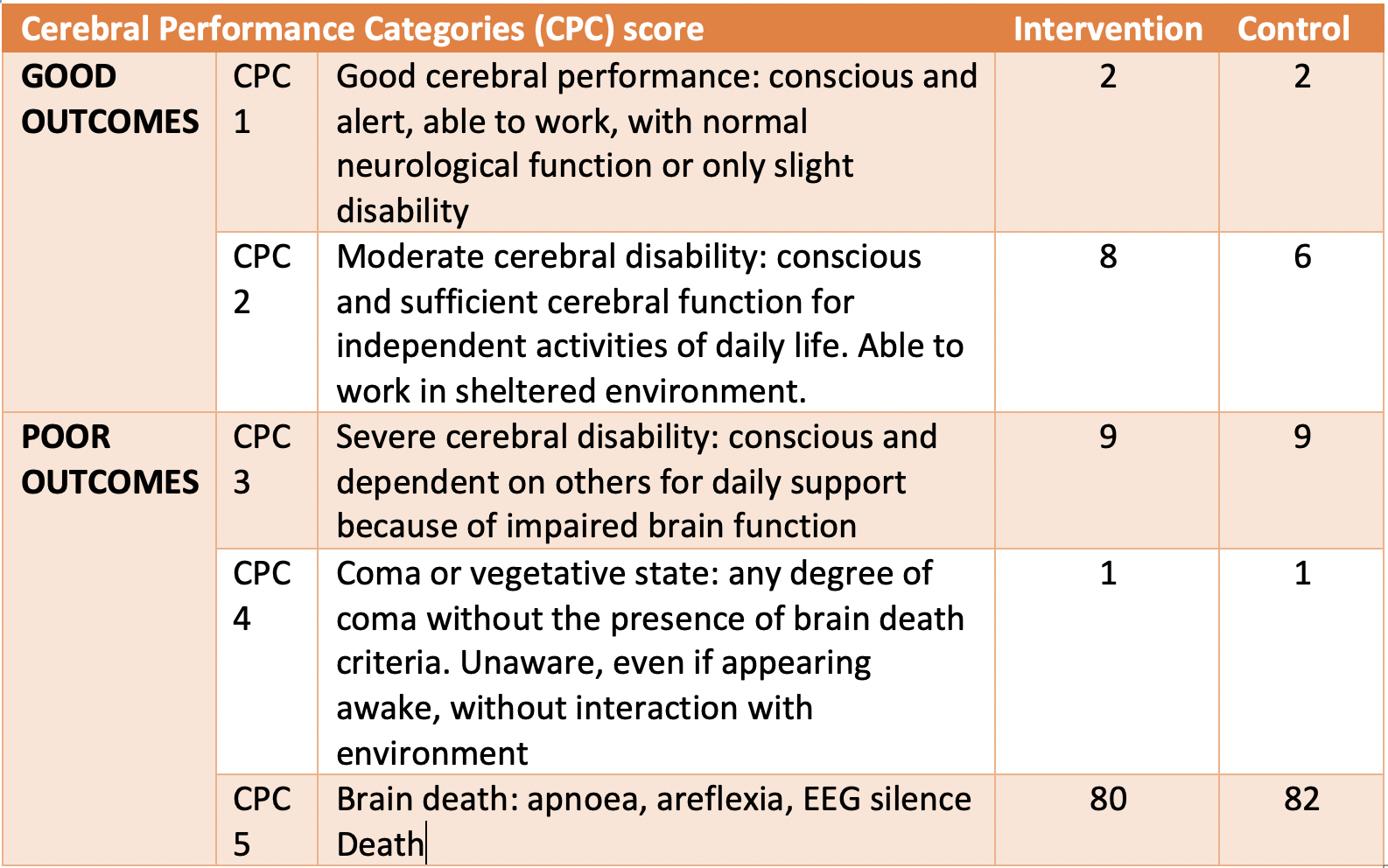
- Secondary outcomes [comparing intervention vs control]:
- Death: no difference 80% vs 82%
- However, within 24 hours of randomisation, 9% patients in the antiseizure group had died compared with 24% in the control group with withdrawal of life-sustaining therapies occurring in 2% of the antiseizure group compared with 13% in the control group
- Mean LOS in ICU: longer in intervention group
- 8.7 vs 7.5 days
- Mean duration of mechanical ventilation: longer in intervention group
- 7.8 vs 6.6 days
- Death: no difference 80% vs 82%
- Per protocol analysis: similar results
- Subgroup Analyses: no significant difference in any pre-specified subgroup (type of EEG activity, background EEG activity, onset of activity post cardiac arrest)
Authors’ Conclusions
- In comatose patients after cardiac arrest with rhythmic and periodic EEG activity, intensive antiseizure treatment over a period of at least 48 hours did not improve neurologic outcomes at 3 months
Strengths
- Randomised
- Allocation concealment
- Variable block sizes
- Blinding of assessors
- Complete follow-up
- Intention to treat analysis
- An important clinical question with clinically meaningful outcome measures which also takes into account utilisation of ICU resources
Weaknesses
- 8 patients in control group received antiepileptic drugs, however, the per protocol analysis yielded similar results
- The small sample size has resulted in a wide confidence interval for the primary outcome and therefore a modest benefit or harm is not ruled out
- The unblinded nature of the study may have impacted end of life decision making. Certainly, patients in the control group were more likely to have therapies withdrawn at an earlier stage compared with the intervention group
- The EEG readers were aware of the trial-group assignments
- Continuous EEG monitoring is not routine practice in a lot of ICUs. However, this trial suggests that treating the abnormal patterns seen does not help patient outcomes. This trial does not support a role for routine cEEG.
- The CPC scale may have some inter-rater variability with one study suggesting 22% discordance between scores on the five point scale and 10% discordance in dichotomised scoring
The Bottom Line
- Treating rhythmic and periodic EEG patterns with intensive antiseizure therapies in comatose survivors of cardiac arrest does not improve mortality and likely prolongs the intensity of therapies without benefit to neurological recovery
- I would not advocate to treat abnormal EEG findings with antiseizure therapies in this patient group
External Links
- [article] Treating Rhythmic and Periodic EEG Patterns in Comatose Survivors of Cardiac Arrest
- [editorial] Futility of Suppressing Seizure-like Activity in Postresuscitation Coma
- [further reading] Introduction to interpretation of the EEG in intensive care
- [further reading] Inter-rater reliability of post-arrest CPC scores
Metadata
Summary author: @celiabradford
Summary date: 5th March 2022
Peer-review editor: George Walker
Picture by: iStock




There is a typo in the exclusion criteria of the telstar trial; exclusion GCS<3 prehospital should be GOS (glascow outcome scale). It would be very strange to exclude comatose patients. I just checked the supplement.