VITAMINS
Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock
Fujii. JAMA 2020; Published online January 17, 2020. doi:10.1001/jama.2019.22176
Clinical Question
- Does treatment with vitamin C, hydrocortisone, and thiamine lead to a more rapid resolution of septic shock compared with hydrocortisone alone?
Background
- Sepsis is life-threatening organ dysfunction due to a dysregulated host response to infection
- A study published in the Lancet shows that in 2017, an estimated 48·9 million incident cases of sepsis were recorded worldwide and 11·0 million sepsis-related deaths were reported, representing 19·7% of all global deaths
- High-dose intravenous (IV) vitamin C has recently been explored as an adjunctive therapy in sepsis because of its anti-inflammatory and antioxidant properties. An RCT (Fowler, 2014) of 24 patients showed that high-dose IV vitamin C attenuated organ failure associated with sepsis in a dose-dependent manner. Thiamine deficiency has also been reported in 20% of critically ill patients with sepsis (Donnino 2010), and thiamine supplementation has been shown to improve lactate clearance in patients with sepsis (Woolum 2018)
- The combination of high-dose IV vitamin C and hydrocortisone together with thiamine was assessed in a single-center retrospective before-and-after study of 94 patients with severe sepsis or septic shock (Marik 2017). The intervention was associated with shorter duration of vasopressor administration and lower hospital mortality
Design
- Pilot, feasibility, multi-centre, randomised, open-label, phase IIb clinical trial
- Random allocation sequence was generated at the coordinating center using computer-generated random numbers with permuted block sizes of 2, 4, and 6 in a 1:1 ratio stratified by site
- Sample size was initially calculated from a retrospective before and after study with 126 patients required based on an SD of 42 vasopressor-free hours up to day 7. This was then updated from the pooled SD for the first 60 patients enrolled in the study, and the required sample size was recalculated.
- Based on an updated SD of 51.6 hours, the study was estimated to require 180 patients to have 90% power (2-sided α = .05) to detect a difference in vasopressor-free hours of 25
- As the distribution of the primary outcome was expected to be nonparametric, and nonparametric tests have been shown to have decreased statistical power compared with parametric tests, the sample size was inflated by 15% (189 patients). Finally, to account for consent withdrawal (5%), a total of 216 patients were planned to be enrolled.
Setting
- 10 intensive care units in Australia, New Zealand, and Brazil
- May 2018 to July 2019
Population
- Inclusion: Patients admitted to a study intensive care unit (ICU) with a primary diagnosis of septic shock based on the Sepsis-3 consensus definition. This involves ALL of the following criteria being met:
- suspected or documented infection
- acute increase of at least 2 points in the Sequential Organ Failure Assessment (SOFA) score
- lactate level greater than 2 mmol/L
- vasopressor dependence for at least 2 hours at the time of enrollment
- Exclusion: age younger than 18 years, a do-not-resuscitate order, imminent death, diagnosis of septic shock longer than 24 hours ago, known or suspected disease with a strong indication or contraindication for any of the study drugs, and another indication for hydrocortisone than septic shock
- 786 patients were screened; 216 were randomised; 5 patients (2 in the intervention group and 3 in the control group) either withdrew or refused consent to continue participation and withdrew all data, leaving 211 patients
- At baseline, patients in the intervention group had lower APACHE III scores, had higher lactate and white blood cell counts, and were more likely to have received milrinone. The primary sites of infection were predominantly pulmonary and gastrointestinal in the two groups
- Mean age, 61.7 years
- 133 men [63.0%] and 78 women [37.0%]
Intervention
- Received IV vitamin C (1.5 g every 6 hours), hydrocortisone (50 mg every 6 hours), and thiamine (200 mg every 12 hours)
- The study intervention continued until cessation of vasopressor administration or when any of the other criteria for stopping the study intervention were met. These were:
- Shock resolution; defined as when all vasopressors were discontinued for four consecutive hours in the presence of a mean arterial pressure (MAP) >65 mmHg or a target MAP set by the treating clinician
- 10 days of vitamin C and thiamine has been administered in the Vitamins group
- 7 days of hydrocortisone and tapering, if applicable, has been delivered in the Control group
- Death
- Discharge from the ICU
- Contraindications to any of the study drugs has arisen
- Serious adverse events suspected to be related to a study medication has developed
- Consent withdrawn or consent to continue not granted
Control
- Received IV hydrocortisone (50 mg every 6 hours)
- thiamine administration was allowed at the discretion of attending ICU clinicians but not vitamin C
Outcome
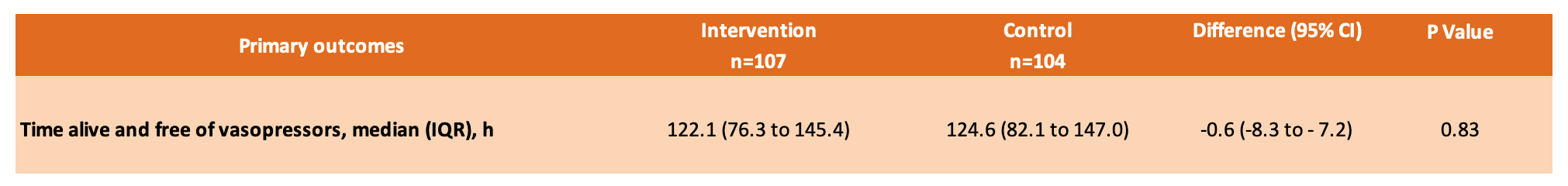
- Primary outcome: There was no significant difference in the median time alive and free of vasopressors up to day 7 after randomisation between the intervention group and the control group
- 122.1 hours [IQR, 76.3-145.4] vs 124.6 hours [IQR, 82.1-147.0]
- Median difference between groups, –0.6 hours [95% CI, –8.3 to 7.2]; P = .83
Time alive and free of vasopressors meant the patient was alive at discontinuation of all intravenous vasopressors for at least 4 hours in the presence of a MAP>65 mmHg or target MAP set by treating physicians. If a patient died while receiving vasopressor therapy following the index episode of septic shock, the patient was assigned zero hours for this outcome. If a patient was weaned from all vasopressors for 4 consecutive hours, then all of the remaining time through day 7 was treated as success, even if the patient died or had vasopressors restarted after weaning within the 7-day period
Use of vasopressors defined as any use of norepinephrine, epinephrine, vasopressin, (*metaraminol, dopamine, or phenylephrine). Total vasopressor doses was calculated as the sum of norepinephrine doses and epinephrine and vasopressin doses were converted to equivalent norepinephrine doses. *Patients receiving metaraminol monotherapy did not contribute to total vasopressor dose data, and no patients received dopamine or phenylephrine
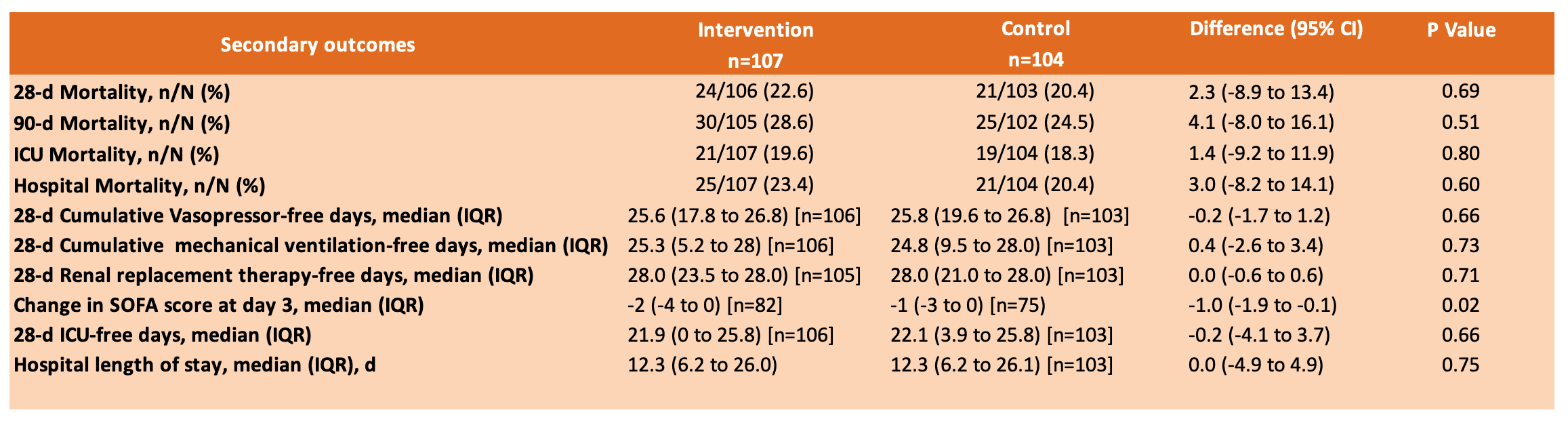
- Secondary outcomes: Of 10 pre-specified secondary outcomes, only one (change in SOFA score at day 3) showed a statistically significant difference
- Adverse Events: reported for 2 patients (2 events, fluid overload and hyperglycemia) in the intervention group and 1 patient (1 event, gastrointestinal bleeding) in the control group. No serious adverse events or suspected unexpected serious adverse reactions were reported
Authors’ Conclusions
- In patients with septic shock, treatment with intravenous vitamin C, hydrocortisone, and thiamine, compared with intravenous hydrocortisone alone, did not significantly improve the duration of time alive and free of vasopressor administration over 7 days. The finding suggests that treatment with intravenous vitamin C, hydrocortisone, and thiamine does not lead to a more rapid resolution of septic shock compared with intravenous hydrocortisone alone
Strengths
- Clinically important question with relevant patient outcomes measured
- A priori publication of trial protocol and statistical analysis plan
- Reason for a change in sample size based on power analysis is clearly explained in the manuscript
- Conducted at 10 sites including high and middle-income countries
Weaknesses
- Open label and lacked blinded outcome assessment, thus creating the possibility of performance and ascertainment bias
- Effects of vitamin C and thiamine were not assessed separately
- Thiamine levels were not measured in the trial, making it uncertain whether randomised patients did or did not have thiamine hypovitaminosis at randomisation and whether this was corrected
- Target MAP set for each patient by treating clinicians was not collected
- Trial was underpowered to detect differences in mortality or other patient-centered outcomes
- Adverse events were reported only when treating clinicians adjudicated, and patients were not systematically examined for other possible adverse effects (eg, oxaluria) that might develop with high-dose IV vitamin C
- There is no data relating to fluid balance or cardiac output monitoring which may have influenced vasopressor requirement
- Time to the administration of antibiotics was not collected
- The median time from ICU admission to randomisation was 12 hours. Even if delays presenting to Emergency Department and transferring to ICU are not taken into account, this delay may have reduced drug efficacy
The Bottom Line
- I will continue my current practice which is not to use the HAT (hydrocortisone, ascorbic acid and thiamine) protocol for treating septic shock
- Further studies looking at timing of treatment intervention may be helpful although the unintended consequences of diverting funding from other research opportunities in sepsis and other diseases must also be considered. Completion of several existing trials will hopefully provide the information we need
- Whilst we continue our search for the elusive magic bullet for sepsis we must not lose focus on interventions that we know are life saving: the right antibiotics given early, together with antimicrobial stewardship, source control, and optimal physiological support
External Links
- [article] Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock. The VITAMINS Randomized Clinical Trial
- [further reading] Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020
- [further reading] Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014
- [further reading] Effect of Thiamine Administration on Lactate Clearance and Mortality in Patients With Septic Shock. Crit Care Med 2018
- [further reading] Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017
- [blog] The Vitamins trial. Hydrocortisone, Vit C and Thiamine (Marik protocol – or not?) in sepsis. St Emlyn’s
- [blog] VITAMINS Trials – Vitamin C + Hydrocortisone + Thiamine in Septic Shock. FOAMcast
- [blog] PulmCrit- Metabolic Resuscitation: Was the answer inside us all along?
VITAMINS Trial Result from Critical Care Reviews
Metadata
Summary author: Steve Mathieu
Summary date: 19th January 2020
Peer-review editor: Adrian Wong




Well done Steve
A few comments
Re: “ Trial was underpowered to detect differences in mortality or other patient-centered outcomes” – this study was not powered to detected a minimally meaningful ARR in mortality (eg 1%) but had more than enough power to detect the mortality benefit claimed following the Marik 2017 before-and-after study.
Re: timing. I have so far been unable to find any published data supporting the importance of timing, though the assumption that urgent treatment for a time critical illness has “face validity” (ie seems to make sense). Dr Fowler’s team in the CITRIS-ALI didn’t seem to put importance on the timing of Vit C related to onset of sepsis – they sometimes had to wait days for the PFratio to drop <300, which was needed for inclusion in the study. Furthermore, the onset of sepsis can be well before hospital presentation.
The burden of proof is on proponents of HAT therapy to produce robust scientific evidence supporting the importance of timing.
Unfortunately, given the complete lack of a signal of benefit in this phase 2 study, there may not be interest in pursuing it further.
Your bottom line is spot on!
Good Steve
This study was not powered to detected a minimally meaningful ARR in mortality (eg 1%) but had more than enough power to detect the mortality benefit claimed following the Marik 2017 before-and-after study.