LOVIT Trial

Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit
Lamontagne F, @LamontagneFran5, et al. N Engl J Med 2022. DOI: 10.1056/NEJMoa2200644
Clinical Question
- In adult patients with sepsis receiving vasopressor therapy, does a high dose of vitamin C in comparison to placebo, reduce the risk of death or persistent organ dysfunction at 28 days?
Background
- In sepsis, the antioxidant effects of vitamin C therapy may mitigate tissue injury induced by oxidative stress
- A single center study published in 2017 suggested that the early use of intravenous vitamin C, together with corticosteroids and thiamine, were effective in preventing progressive organ dysfunction, and in reducing the mortality of patients with severe sepsis and septic shock, which spurred an interest in Vitamin C
- The CITRIS-ALI Trial demonstrated that high dose Vitamin C did not significantly reduce organ failure scores or improve biomarker levels, in patients with sepsis and ARDS
- Recent meta-analysis showed Metabolic resuscitation with vitamin C, glucocorticoids, vitamin B1, or combinations of these was not significantly associated with a decrease in longer-term mortality
- Although another meta-analysis suggested IV vitamin C administration appears safe and may be associated with a trend towards reduction in overall mortality
Design
- International multicenter, Concealed-Allocation, Parallel-Group, Blinded, Randomized Controlled Trial
- Living umbrella RCT
- Randomized 1:1 using centralized Web based system using permuted blocks of variable, undisclosed size and stratified according to the site
- Written informed consent
- Completely blinded except for on site pharmacist who prepared the solution
- Based on a power of 80% and Type 1 error of 5%, a sample size of 385 selected for each group
- To account for withdrawal of consent and loss to follow-up, plan to enroll 400 patients per group was made
- Allocation further inflated to include COVID Patients
Setting
- 35 adult medical–surgical ICUs in Canada, France, and New Zealand
- Patients enrolled From November 14, 2018, to July 19, 2021
Population
- Inclusion:
- Patients ≥18 years old
- Admitted to ICU with proven or suspected infection as the main diagnosis
- Currently treated with a continuous intravenous infusion of vasopressors (norepinephrine, epinephrine, vasopressin, dopamine, phenylephrine)
- Exclusion:
- >24 hours of intensive care unit (ICU) admission
- Known Glucose-6-phosphate dehydrogenase (G6PD) deficiency
- Pregnancy
- Known allergy to vitamin C
- Known kidney stones within the past 1 year
- Received any intravenous vitamin C during this hospitalization unless incorporated in parenteral nutrition
- Expected death or withdrawal of life-sustaining treatments within 48 hours
- Previously enrolled in this study
- Previously enrolled in a trial for which co-enrolment is not allowed (co-enrolment to be determined case by case)
- 2234 screened –> 872 randomized ->8 patients further found to be ineligible ->1 further withdrew consent
- The actual number of patients who completed follow up at 28 days were
- 429 in Vitamin C group
- 434 in Placebo group
- Comparing baseline characteristics of Vitamin C vs. Placebo group
- Age(yrs): 65.0 vs 65.2
- Female Sex (%): 35.2 vs 40
- Admission type
- Medical (%) :81.6 vs 85.2
- Surgical (%): 18.4 vs 14.8
- APACHE II Score: 24.2 vs 24.1
- SOFA score: 10.2 vs 10.1
- Primary site of infection (%)
- Pulmonary: 33.8 vs 36.7
- Gastrointestinal or intra-abdominal: 31 vs 25.9
- SARS CoV-2 Positive (%): 8.6 vs 6.0
- Lactate mmol/liter: 3.4 vs 3.0
- Vitamin C level µmol/liter: 20.6 vs 19.1
- Septic shock (requirement for a vasopressor infusion and a lactate >2 mmol per liter) (%): 59.6 vs 56.1
- Time from ICU admission to randomization (hr): 12.9 vs 12.3
- Treatment (%)
- Glucocorticoids: 46.4 vs 45.4
- Mechanical ventilation: 68.5 vs 65.4
- Renal Replacement therapy: 10.7 vs 9.7
- Vasopressor infusion: 99.8 vs 100
Intervention
- High dose vitamin C
- 50 mg per kilogram actual body wt mixed in a 50-ml solution of either dextrose 5% in water or normal saline
- Administered over 30 to 60 minutes every 6 hours for 96 hours (i.e., 200 mg per kilogram per day, with a maximum of 16 doses) as long as patients remained in the ICU
Control
- Placebo
- Received a matching placebo infusion (dextrose 5% in water or normal saline)
Management common to both groups
- None specified
- All co-interventions including glucocorticoids and thiamine were performed at the discretion of treating teams and were recorded
Outcome
- Primary outcome:
- Composite of death or persistent organ dysfunction (defined as receipt of vasopressors, invasive mechanical ventilation, or new renal-replacement therapy) on trial day 28 – significantly higher in Vitamin C group
- Vit C 191/429 (44.5%) vs Placebo 167/434 (38.5%)
- Risk ratio, 1.21; 95% confidence interval [CI], 1.04 to 1.40; P = 0.01
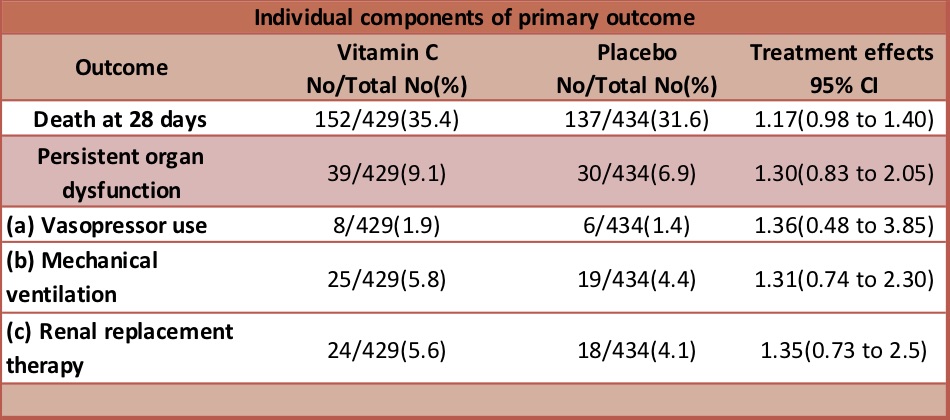
- Secondary outcomes:
- No significant difference in:
- Median no. of days without organ dysfunction in ICU at day 28 (Vitamin C 17 vs 19.5 Placebo)
- Death by 6 months no/total (%): 191/417(45.8) vs 185/426(43.4)
- Health related Quality of life at 6 months
- SOFA scores on day 1,2,3,4,7,10,14,28
- Markers of tissue dysoxia (Lactate), inflammation and endothelial injury at day 1,3 and 7
- No significant difference in safety outcomes
- Stage 3 AKI
- Acute haemolysis
- Hypoglycemia
- No significant difference in:
Authors’ Conclusions
- In adults with sepsis who were receiving vasopressor therapy in the ICU, the administration of intravenous vitamin C resulted in a higher risk of death or persistent organ dysfunction at 28 days than the receipt of placebo
Strengths
- Largest RCT on topic to date
- Well-designed international trial
- The primary outcome in this trial, a composite of death or persistent organ dysfunction, included death to address the competing-risk issue
- Vitamin C had a consistent effect across all elements of the composite outcome and on 6-month mortality
- Increased statistical efficiency
- Blinding to limit ascertainment bias
- Median enrollment time of approximately 12 hours after ICU admission
- Protocol adherence
- Assessment of biomarkers and baseline vitamin C levels
Weaknesses
- Nine patients after randomization did not contribute data
- Information regarding specific pathogens and antimicrobial therapy not collected
- Information to ascertain the presence of acute respiratory distress syndrome at baseline was not collected
- Patient population from ICU of high-income countries which differs substantially from low- and middle-income countries, where incidence of sepsis and case fatality rate are highest
The Bottom Line
- I will not use high dose Vitamin C in patients with sepsis on vasopressors as a number of trials and a recent meta-analysis have shown no benefit
- This trial should end the debate regarding use of Vitamin C in septic shock patients
External Links
- Article Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit
- Further reading Parenteral Vitamin C in Patients with Severe Infection: A Systematic Review
Metadata
Summary author: Kirti Vashist
Summary date: 10th July 2022
Peer-review editor: David Slessor
Image by: Vino Li on Unsplash




What are your thoughts on the LOVIT trial including 57 patients in the IVC group that were transferred from another ICU where they had already been in for an average of 49 hours (with standard deviation of 76 hours)? See Table S1 of the supplemental appendix.