MIDAS

Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial
Santer P et al. Intensive Care Medicine. 2020. DOI: 10.1007/s00134-020-06216-x
Clinical Question
- In patients aged > 18 years who were unable to be liberated from vasopressors for 24 hours, did the addition of midodrine when compared to a placebo result in the earlier discontinuation of vasopressors?
Background
- Midodrine is an oral alpha-1 adrenergic agonist licensed to treat orthostatic hypotension
- It has been used in ICU off-label in order to try and liberate patients from vasopressors
- They have been no randomised clinical trials studying the use of midodrine in this setting
Design
- Multi-centre, randomised, double-blind, placebo-controlled trial
- Randomised in 1:1 ratio using computer generated sequence stratified by site
- List provided directly to compounding pharmacy
- Power calculations required 50 patients in each arm to detect a 6 hour difference in time to vasopressor discontinuation (two sided alpha of 0.05, power 80%)
- Power calculations based on a study that involved 20 surgical patients looking at the rate of decline of IV phenylephrine (or phenylephrine equivalent) administration following midodrine use
- Analysis performed using modified intention to treat approach
- All those who included one dose of the drug were analysed
- Written consent provided for all participants
- Approved by local boards at each site and registered with US FDA and Australian TGA
Setting
- 3 tertiary referral centres:
- 2 in Boston, USA and 1 in Perth, Australia
- Data collected from October 2012 – June 2019
Population
- Inclusion:
- Aged > 18 years
- Single agent vasopressor at time of randomisation
- Doses had to be < 100 mcg/min phenylephrine, < 8 mcg /min norepinephrine or < 60 mcg/min metaraminol
- If multiple vasopressors and/or doses greater than those listed had been used, patients were still eligible as long as criteria above met at time of randomisation
- Patients had to be resuscitated and reversible causes of hypotension treated
- Unable to be liberated from vasopressors for > 24 hours
- Exclusion:
- Clinical evidence of inadequate tissue oxygenation
- Hypovolaemic shock
- Hypotension due to adrenal insufficiency, liver failure, chronic renal failure or severe cardiac failure (LVEF < 30%), acute urinary retention, pheochromocytoma, thryotoxicosis, bradycardia (HR < 50)
- Pregnancy
- Received midodrine prior to enrollment or known allergy to midodrine
- Inability to receive enteral medications
- Enrolled in another trial
- 136 patients randomised
- 530 assessed, 213 eligible
- 317 excluded (193 did not meet inclusion criteria, 124 met exclusion criteria)
- 68 randomised in each arm
- 66 analysed in each arm
- 2 patients in each arm had no vasopressor requirement prior to drug administration so did not receive any midodrine / placebo
- 530 assessed, 213 eligible
- Baseline characteristics similar, comparing midodrine vs. placebo group
- Male: 55% vs. 9%
- Age 70 vs. 67
- White/Caucasian: 97% vs. 94%
- APACHE II score: 14.7 vs. 14.8
- Indication for ICU admission
- Post-op/surgical: 68% vs. 64%
- Sepsis: 20% vs. 20%
- Medical/other: 12% vs. 17%
- Epidural analgesia: 27% vs. 20%
- Duration of vasopressor administration before study drug administration: 35.5 vs. 35.4 hours
- Baseline MAP: 76 vs. 73mmHg
- Vasopressor dose at enrollment (mcg/kg/min)
- Norepinephrine (n=41): 0.06 vs. 0.06
- Phenylephrine (n=28): 0.61 vs. 0.43
- Metaraminol (n=60): 0.6 vs. 0.61
Intervention
- Midodrine
- 20 mg oral
- Administered 8 hourly
Control
- Placebo
- Matched in appearance
- Administered orally 8 hourly
Management common to both groups
- Drugs administered until:
- ICU discharge
- BP goal met for > 24 hours without vasopressors (trial drug could then be ceased at discretion of treating clinicians)
- Worsening hypotension requiring high dose vasopressors
- Requirement for adrenaline
- Signs and symptoms of organ dysfunction, or hypo-perfusion
- Adverse events related to midodrine
Outcome
- Primary outcome:
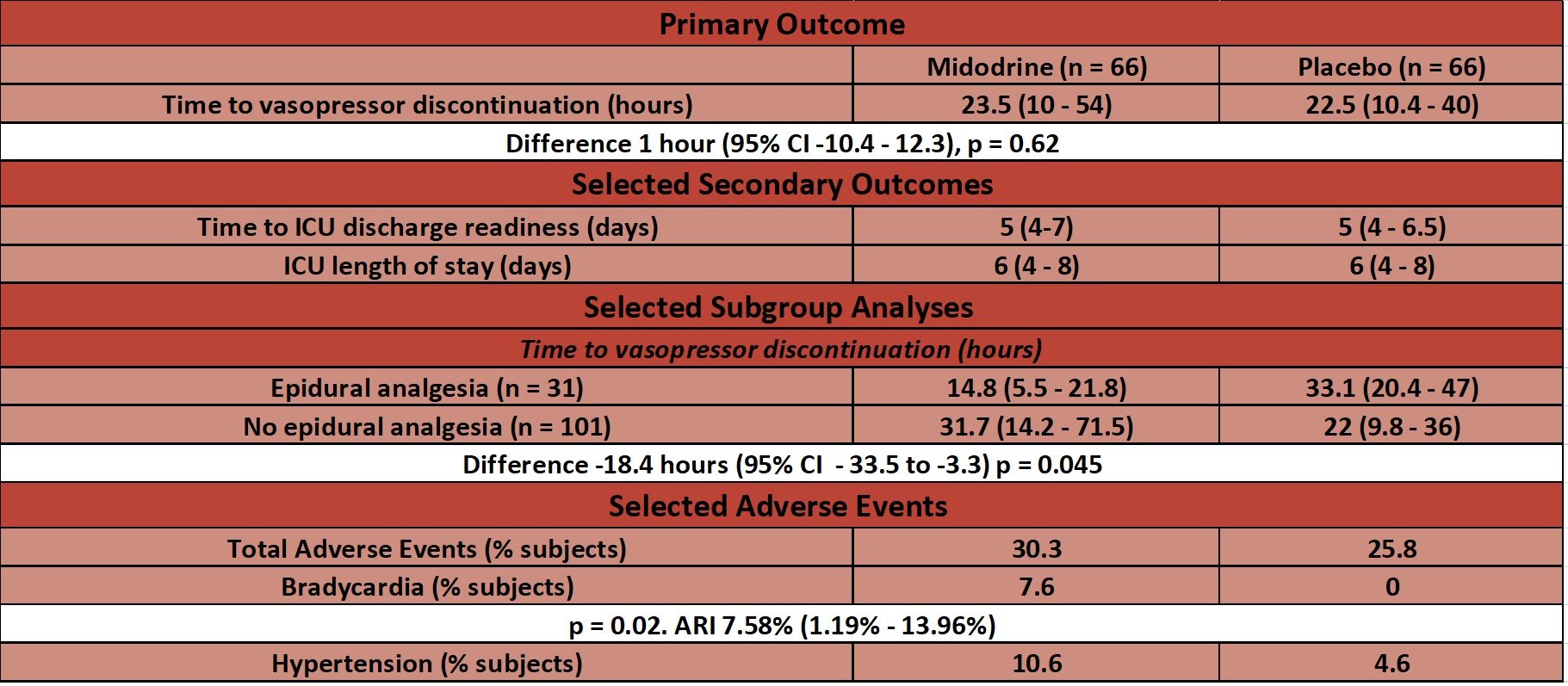
- Median time from study drug initiation until discontinuation of IV vasopressors – no significant difference
- 23.5 hours (IQR 10 – 54 hours) in midodrine group vs. 22.5 hours (IQR 10.4 – 40 hours) in placebo
- Difference 1 hour (95% C.I. -10.4 to 12.3, p=0.62)
- Discontinuation defined as a vasopressor free period of 24 hours
- Median time from study drug initiation until discontinuation of IV vasopressors – no significant difference
- Secondary outcomes:
- No statistical difference in time to readiness for ICU discharge, ICU length of stay (LOS), hospital LOS and ICU readmission
- Post-Hoc Subgroup Analyses for primary outcome – time to vasopressor discontinuation
- In patients with epidural analgesia – significantly reduced in midodrine vs. placebo group
- Difference -18.4 hours (95% C.I. -33.5 to -3.3, p=0.045)
- In patients without epidural analgesia – no significant difference
- Difference 9.7 hours (95% C.I. -6.3 to 25.7, p=0.1)
- No differences found based on the following subgroups: ICU admission reason or opioid usage
- Opioid usage analysed to address possibility of impaired GI absorption
- In patients with epidural analgesia – significantly reduced in midodrine vs. placebo group
- Adverse Events:
- Bradycardia significantly more common in midodrine group
- 8% vs. 0%, p=0.02
- Bradycardia defined as HR < 40, or decrease in HR > 20% from specified goal
- Bradycardia significantly more common in midodrine group
Authors’ Conclusions
- The use of midodrine did not result in earlier liberation from vasopressors in critically ill adults when compared to a placebo
Strengths
- Randomised, double blind and placebo controlled increases internal validity
- Multi-site and international
- The inclusion criteria of a low dose, single vasopressor usage seems sensible given the postulation that the administration of midodrine may help in those patients with a stubborn, low dose vasopressor requirement
- Inclusion criteria broad which means applicable to a wide range of ICU patients
- However as the authors allude (especially given the small sample size) this might mean the sample population is too heterogeneous to show effect
- This may be highlighted by the results of those with epidural analgesia
- They authors rightly don’t draw any inference from this under-powered, post-hoc sub group analyses but suggest this finding is hypothesis generating
- Sensible inclusion of safety outcomes in analysis
- No data for analysis of primary outcome was missing
Weaknesses
- The dosing requirements for inclusion didn’t specify a weight based dose
- 8mcg / min norepinephrine in a 45kg patient equates to 0.18mcg/kg/min compared to 0.06mcg/kg/min in a 120kg patient
- When reviewing the supplementary material:
- Baseline MAP for the midodrine group was 75.9 mmHg and for the placebo group was 72.8 mmHg
- 24 hours following study drug initiation these had only increased by 1.3 mmHg (midodrine group) and 1.5 mmHg (placebo group)
- Baseline MAP seems higher than one would conventionally target when patient remains on a small vasopressor dose. Could discontinuation have occurred by altering the MAP target (ie. to 65mmHg) without the need for midodrine?
- Mean fluid balance 100 mls higher in midodrine group than placebo on day 1 of study drug (+927 mls vs +826mls)
- No further details surrounding fluid management is given
- Baseline MAP for the midodrine group was 75.9 mmHg and for the placebo group was 72.8 mmHg
- Despite being multi-site and international, there were only three sites from two countries, both of which are high income countries which may minimise external validity
- 64% (n = 85) of patients were randomised from the Australian site
- Small sample size
- 66 % (n = 87) post-operative / surgical
- Does a post operative vasoplegic state resolve differently to other vasoplegic / vasodilatory states?
- 66 % (n = 87) post-operative / surgical
- There is no data provided as to the requirement of other organ supports for these patients
- If a patient’s vasopressors are ceased earlier but they remain intubated then the outcome of faster vasopressor cessation is probably not clinically relevant
- Why did recruitment take so long (7 years) in a multi centre trial when the main inclusion criteria was requirement > 24 hours of low dose vasopressors?
- This suggests there may have been a selection bias
- Additionally, the exclusion of patients already receiving midodrine (n=24) may also lead to a selection bias
- Subgroup analyses not pre-specified
The Bottom Line
- This RCT in patients with an ongoing need for low dose vasopressors, reported that the use of midodrine compared with placebo, did not result in any significant reduction in the time to the discontinuation of IV vasopressors
- I will not be using midodrine to help liberate patients from vasopressors, especially with increased rate of bradycardic events
- Although “non-significant”, this trial is important in advancing clinical care given the limited prior evidence on the use of midodrine in this manner.
External Links
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 9th September 2020
Peer-review editor: @davidslessor



