TRAAP2
TRAAP2: Tranexamic Acid for the Prevention of Blood Loss after Cesarean Delivery
Sentilhes L. New Eng J Med 2021; 384:1623-1634. doi:0.1056/NEJMoa2028788
Clinical Question
- In patients undergoing cesarean section (CS) does the administration of tranexamic acid (TXA) when compared to a placebo result in a lower incidence of calculated estimated blood loss or red blood cell transfusion by day 2?
Background
- The use of TXA has been widely studied in post partum haemorrhage (PPH)
- The WOMAN trial showed that TXA may be beneficial in reducing the risk of death due to PPH
- This has been lauded as a landmark trial but there are some controversies that are excellently summarised here
- The evidence surrounding the use of TXA post CS is limited and consists mainly low quality evidence and there is no strong evidence to support its use in a prophylactic manner
Design
- Multi-centre, randomised, placebo controlled, double blind trial
- Randomised to receive uterotonic agent plus either TXA or placebo
- Randomised in 1:1 ratio
- Computer based, in blocks of 4
- Stratified by site and whether CS done pre or during labour
- TXA and placebo in identically labelled packaging and vials
- Appropriate consent process – written informed consent obtained by treating teams if CS considered likely
- Blood loss estimated using following equation:
- Estimated blood volume x [(pre-op haematocrit-post-op haematocrit) / post-op haematocrit]
- Pre-op haematocrit was most recent in 8 days prior to caesarean
- Post-op was closest to day 2 post delivery
- Physician responsible for the delivery prospectively recorded the procedures used during the third stage of labor and clinical outcomes identified in the immediate postpartum period
- Adverse events were assessed until hospital discharge and a telephone interview was conducted at 3 months post delivery
- 4525 patients required
- 4072 patients estimated (80% power to detect a 20% relative difference in incidence of primary outcome, with 5% type 1 error rate)
- Allowed for up to 10% to be lost to follow, deliver vaginally or not have blood tests taken on day 2
- Predominantly based on data from ECSSIT trial
- Performed with modified intention to treat population
- Those who withdrew consent, delivered vaginally, or deemed ineligible after randomisation were excluded
- There were two pre-specified pre-protocol populations: those who received TXA within 3 minutes and those who received TXA within 10 minutes
- Registered with clinicaltrials.gov NCT3431805
Setting
- 27 centres in France
- March 2018 – January 2020
Population
- Inclusion:
- > 18 yo
- >/= 34 weeks gestation
- expected to undergo Caesarean before or during labour
- Exclusion:
- Known or suspected increased risk of VTE / arterial embolism
- Increased risk of bleeding (including administration of LMWH or anti-platelet in week prior)
- History of seizures or epilepsy
- Eclampsia
- Autoimmune disease
- Failed operative vaginal delivery
- Sensitivity to trial drugs
- Hb < 9g/dL in the week prior
- Poor comprehension of spoken French
- 4551 randomised
- 2276 – TXA
- 2275 – placebo
- Baseline demographics similar
- Age: 33.3 (TXA) vs 33.5 (Placebo)
- BMI: 26.3 vs. 26.1
- Primip: 37.2% vs. 36.6%
- Prior CS: 51.8% vs. 52.4%
- History of PPH: 5.1% vs. 4.5%
- Multiple pregnancies: 7.2% vs. 7.2%
- Timing of CS – during labour: 28.9% vs. 29.2%
- GA: 3.0% vs. 3.8%
- Prophylactic uterotonic: 98.9% vs. 99.0%
- Anticoagulant prophylaxis post delivery: 58.8% vs. 59.1%
- Median duration of interval from birth to TXA: 2 mins vs. 2 mins
Intervention
- 1g TXA
- 10ml vial
Control
- Normal saline
- Identically labeled vial
Management common to both groups
- Either TXA or placebo given over 30-60 seconds in the first three minutes after birth
- This was given after bolus prophylactic uterotonics given (5-10u oxytocin or or 100 μg of carbetocin)
- Subsequent oxytocin infusion at discretion of treating unit
- All spent at least 2 hours in PACU / Recovery until bleeding diminished to expected amount
Outcome
- Primary outcome:
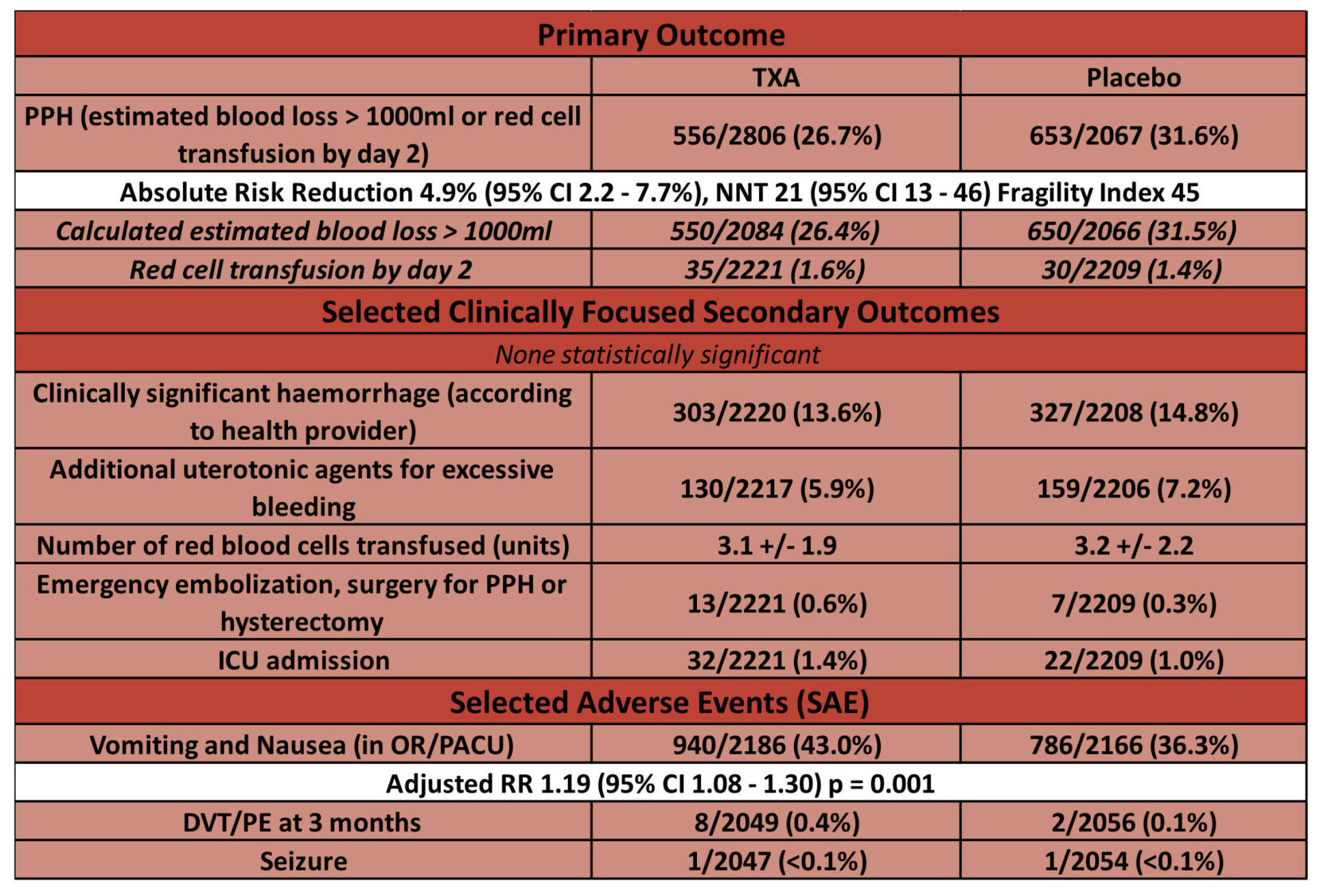
- Postpartum hemorrhage (defined as a calculated estimated blood loss greater than 1000 ml or red-cell transfusion by day 2): – significantly reduced in tranexamic acid group
- 556/2086 (26.7%) TXA vs. 653/2067 (31.6%) Placebo
- Adjusted RR 0.84 (95% CI – 0.75 – 0.93, p = 0.003)
- No difference between pre-specified subgroups (PPH risk and timing of CS)
- Secondary outcomes:
- No significant differences in:
- Gravimetrically estimated blood loss: 689ml vs. 719ml
- Estimated by suction volume and swab weight
- Clinically significant blood loss: 13.6% (TXA) vs. 14.8% (Placebo)
- Additional uterotonic agents for excessive bleeding: 5.9% vs. 7.2%
- Blood transfusion: 1.9% vs. 1.8%
- Number of units transfused: 3.1 vs. 3.2
- Gravimetrically estimated blood loss: 689ml vs. 719ml
- Significant difference in pre and post-operative Hb:
- Peripartum change -1.2 g/L vs. -1.4g/L (p < 0.001)
- No significant differences in:
- Adverse Events:
- In PACU or OR:
- Higher rates of nausea and vomiting
- 43.0% vs. 36.3% (p < 0.001)
- Higher rates of nausea and vomiting
- D2 after delivery:
- No significant differences between groups for urea, creatinine, AST, ALT
- Up to 3 months after delivery:
- No significant difference for:
- Seizure: <0.1% vs. <0.1% (1 in each arm)
- DVT/PE: 0.4% vs. 0.1% (8 cases in TXA arm, 2 in placebo)
- No significant difference for:
- In PACU or OR:
Authors’ Conclusions
- The use of TXA in this setting reduced the incidence of PPH (as defined by calculated estimated blood loss > 1000ml or red cell transfusion by day 2).
- However there was no reduction in clinical secondary outcomes
Strengths
- Large, multi-centre trial
- Well balanced balanced baseline characteristics
- High proportions of risk factors for PPH (previous caesarean, prior PPH, obesity, multiple pregnancy, emergency caesarean) present in baseline characteristics
- This is important to ensure that two low risk cohorts not being analysed
- Sensible pre-specified subgroup looking at the use of TXA in those at high risk of PPH vs. low risk
- Defined as presence of a one or more risk factor with an OR > 3
- Good randomisation and blinding strategy results in strong internal validity
- Pragmatic statistical analysis plan
- Although large numbers of women were randomized but not assessed for the primary outcome in the modified intention to treat analysis, the statistical analysis plan allowed for up to 10% drop out following randomisation
- Sensible use of a standardised method of estimating blood loss given clinicians’ estimations are often incorrect
- The values of estimated blood loss obtained by the gravimetrical method (suction volume, and weighing swabs) and the standardised equation similar
- Minimal cross-over / protocol violations
- Only 1.4% in each group received no treatment
- Only 0.1% in each group received the opposite treatment
- Good follow up at three months – important for reporting adverse events
- 93.5% (TXA) vs. 94.4% (Placebo) completed follow up interviews at three months
Weaknesses
- The primary outcome is not patient orientated
- Although the trial is not adequately powered for clinically relevant secondary outcomes there is no suggestion that TXA reduces the rate of these
- The authors rightly acknowledge this in the conclusion
- Although the trial is not adequately powered for clinically relevant secondary outcomes there is no suggestion that TXA reduces the rate of these
- As discussed, the statistical plan allowed for up to 10% drop out from those who are randomised to receive a treatment, however:
- The rates were slightly higher than 10% in each arm:
- TXA – 10.5%
- Placebo – 11.8%
- This may introduce an attrition bias
- 210 (4.6%) patients excluded for unknown reasons or meeting exclusion criteria following randomisation
- This may introduce a selection bias
- 278 (6.1%) had missing data for the primary outcome
- The appendix has a comparison of where only complete cases are analysed (the data presented above) against the imputation of missing values as failures (i.e. they were assumed to have PPH)
- If the missing values are treated as failures then the p value > 0.005
- TXA 31.1% vs Placebo 36.0% p = 0.006
- If the missing values are treated as failures then the p value > 0.005
- The appendix has a comparison of where only complete cases are analysed (the data presented above) against the imputation of missing values as failures (i.e. they were assumed to have PPH)
- The rates were slightly higher than 10% in each arm:
- PPH is a heterogenous process
- The 4Ts is a common acronym for PPH reasons: uterine tone, retained tissue, trauma, thrombin
- TXA does not have a plausible biological mechanism of action in the treatment of PPH for anything other than trauma
- No documentation of suspected reasons for PPH is recorded
- The protocol allowed for the use of a oxytocin infusion as per local hospital policy following the uterotonic bolus
- No mention is made of how many had an oxytocin infusion after
- This could be an important confounder
- No mention is made of how many had an oxytocin infusion after
- The 4Ts is a common acronym for PPH reasons: uterine tone, retained tissue, trauma, thrombin
- The study excluded those who are increased risk of bleeding – would these patients potentially benefit more?
- The BNF suggests that TXA should be given as a slow IV injection (at a rate not exceeding 100mg/minute)
- The protocol states it should be given over 30 to 60 seconds (20x as fast)
- This may be a reason for the increased rate of vomiting and nausea
- The protocol states it should be given over 30 to 60 seconds (20x as fast)
- Approximately 25% did not receive TXA within three minutes in each arm
- This is understandable as it is an already frenetic time ensuring that uterotonics are given, and that the baby and parents are well!
- This delay did not seem to have an effect though as in the protocol analysis which included those who received TXA/placebo within 10 minutes still showed a significant reduction in the primary outcome
The Bottom Line
- The use of prophylactic TXA for caesarean delivery reduces the rates of PPH as defined by an estimated blood loss of > 1000ml or red cell transfusion by day 2
- I will not use TXA in a routine prophylactic manner given this did not seem to show any trend to a reduction in meaningful clinical outcomes, and an increased risk of nausea and vomiting
External Links
Metadata
Summary author: George Walker
Summary date: 9th May 2021
Peer-review editor: David Slessor
Image by: iStock



