PARAMEDIC2

A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest
Perkins G.D. et al. New England Journal of Medicine 2018
DOI: 10.1056/NEJMoa1806842
Clinical Question
- In adult patients with out-of-hospital cardiac arrest (OOHCA), does the administration of epinephrine compared to placebo improve survival?
Background
- There is limited evidence for the drug treatments used in Advanced Life Support (ALS).
- Epinephrine in cardiac arrest has potential beneficial effects by increasing the aortic diastolic pressure and thus increasing coronary blood flow; however harmful effects have also been hypothesised. These include increased myocardial oxygen demand, and platelet activation leading to thrombosis and impaired microvascular flow potentially increasing cerebral ischaemia.
- There has been conflicting evidence as to whether epinephrine (adrenaline) is beneficial or not. A large Japanese observational study reported higher rates of return of spontaneous circulation (ROSC) but worse neurologic outcome. As a result the International Liaison Committee on Resuscitation called for a large-scale RCT to see if epinephrine is safe and effective.
Design
- Randomized, double blinded trial
- Treatment assignments randomly generated using computer
- Allocated in a 1:1 ratio
- Uniquely numbered, identical pre-prepared packs provided to ambulance service containing 10 syringes with either 1mg epinephrine or 0.9% saline
- Ambulance services entered data into a secure portal
- Ethical approval sought and granted
- Funded by Health Technology Assessment Programme of the National Institute of Health Research
- Those funding had no role in design, data collection and analysis or manuscript preparation
- Target sample size calculated at 8,000
- With a risk ratio for the epinephrine group as 1.25 (95% CI is 1.07 – 1.46) this corresponded to a 30-day survival of 6% in placebo group and 7.5% in epinephrine group
- Odd-Ratio adjusted for age, sex, interval between emergency call and ambulance arrival, interval between arrival of ambulance and administration of trial agent, initial rhythm, cause of arrest, whether arrest was witnessed or whether CPR performed by a bystander
- Pre-specified sub-group analyses
- Data monitoring committee (DMC) performed interim reviews every 3 months
Setting
- Conducted by 5 NHS ambulance services in the UK
- December 2014 – October 2017
Population
- Adult Patients > 16 years old presenting with OOHCA in whom ALS had been provided by trial trained paramedics
- Exclusion criteria:
- Pregnancy (known or apparent), cause of arrest was asthma or anaphylaxis, administration of epinephrine prior to arrival of trial trained paramedics
- In one ambulance service (London Ambulance Service), traumatic cardiac arrest was also excluded
- 10,623 screened
- 8,103 eligible (76.3%) and trial packs opened
- Between opening and administration of drug or placebo more information was acquired that indicated in 87 patients there were ineligible
- 2 patients had unknown trial group assignments due to missing trial pack numbers
- 8014 randomized
- 4015 in epinephrine group
- 3999 in control
- 2520 excluded. This included:
- 615 had a return of spontaneous return of circulation
- 1192 had already received adrenaline
- 228 excluded for traumatic cardiac arrest
- 8,103 eligible (76.3%) and trial packs opened
- Similar baseline characteristics in each group
- Age: 69.7 (Epinephrine) vs. 69.8 (Placebo)
- Male: 65.0% vs. 64.6%
- Initial Cardiac Rhythm:
- Shockable: 19.2% vs. 18.7%
- VF: 17.8% vs. 17.1%
- VT: 0.6% vs. 0.5%
- Non-shockable: 78.4% vs. 79.5%
- Asystole: 53.2 % vs. 54.9%
- PEA: 23.8% vs. 23.4%
- Shockable: 19.2% vs. 18.7%
- Cause
- Medical: 91.1% vs. 92.3%
- Traumatic: 1.6% vs. 1.4%
- Drowning: 0.2% vs. 0.3%
- Drug Overdose: 1.8% vs. 1.8%
- Electrocution: 0 vs. <0.1%
- Asphyxia: 2.9% vs. 2.0%
- Missing Data: 2.3% vs. 2.1%
- Witness
- Unwitnessed: 37.3% vs. 37.6%
- Bystander: 50.1% vs. 49.2%
- Paramedic: 11.3% vs. 11.8%
- Missing Data: 1.3% vs. 1.4%
- CPR
- Bystander: 59.3% vs. 58.7%
- Paramedic 11.3% vs. 11.8%
- Missing Data: 1.7% vs. 2.1%
- Time frames for key events also similar
- Median interval between emergency call and ambulance arrival
- 6.7 min (Epinephrine) vs. 6.6 min (Placebo)
- Median interval between emergency call and administration of trial agent
- 21.5 min vs. 21.1 min
- Mean interval between ambulance arrival and departure
- 50.1 min vs. 44.5 min
- Mean interval between ambulance departure and hospital arrival
- 12.9 min vs. 12.4 min
- Medial interval between initiation and cessation of ALS
- 47.5 min vs. 43.1 min
- Mean of 4.9 drug doses given in epinephrine group vs. 5.1 in placebo
- 5% received amiodarone in epinephrine group vs. 9.2% in placebo
- 3980 shocks after randomisation in epinephrine group vs. 3962 in placebo
- Initial Response to Resuscitation
- Return of spontaneous circulation (ROSC) in 36.3% in epinephrine vs. 11.7% in placebo
- Transported to hospital in 50.8% in epinephrine group vs. 30.7% in placebo
- Of those transported 15.3% of epinephrine group not declared dead in emergency room vs. 7.3% in placebo group
- Median interval between emergency call and ambulance arrival
Intervention
- Epinephrine administration as per ALS guidelines
- 1 mg boluses every 3-5 minutes once commenced depending on algorithm being followed
Control
- 0.9% Saline placebo in place of epinephrine (again in accordance with ALS guidelines)
Management common to both groups
- Pre-hospital treatment dictated by ALS algorithm
- Rates of concurrent pre-hospital treatments (IV or IO access, supraglottic airway device, endotracheal tube) similar
Outcome
- Primary Outcome: survival at 30 days was greater in the Epinephrine group compared to the Placebo group
- Epinephrine: 3.2% survived
- Placebo: 2.4% survived
- Absolute Risk Reducion (ARR): 0.89% (95% CI 0.17 to 0.16)
- This is an Absolute Risk Reduction because the intervention ‘reduced’ mortality risk
- This can also be described as an Absolute Risk Increase as the risk of survival has increased
- Adjusted Odds Ratio (OR): 1.47 (95% CI 1.09 to 1.97; P = 0.02)
- Number Needed to Treat (NNT): 112
- Fragility Index (FI): 6
- Secondary Outcomes:
- Survival rates at various time points were significantly better in the Epinephrine group
- Length of stay in ICU and hospital did not differ
- Neurological outcomes were possibly worse in the Epinephrine group
- When categorised into binomial ‘favourable’ and ‘non-favourable’ there was no statistically significant difference
- When assessing the number who survived with severe neurological deficit, the difference was statistically significant with worse outcome in the Epinephrine group
- Bayesian Analysis:
- The posterior probability that epinephrine administration leads to an increase in the absolute rate of survival of at least 1% was 37%
- The posterior probability that epinephrine administration leads to an increase in the absolute rate of survival with a favourable neurological outcome of at least 1% was 1.9%
| Primary Outcome | |||
| Measure | Epinephrine | Placebo | Adjusted Odds Ratio (95% CI) |
| 30 Day Survival | 130/4012 (3.2%) | 94/3995 (2.4%) | 1.47 (1.09 to 1.97) |
| Absolute Risk Increase 0.89% (95% CI 0.17 to 1.61%) | |||
| NNT 112; Fragility Index 6 | |||
| Secondary Outcomes | |||
| Measure | Epinephrine | Placebo | Adjusted Odds Ratio (95% CI) |
| Survival until Hospital Admission | 947/3973 (23.8%) | 319/3982 (8.0%) | 3.83 (3.30 – 4.43) |
| Median ICU Length of Stay of Survivors | 7.5 days (IQR 3.0 – 15.0) | 7.0 days (3.5 – 12.5) | N/A |
| Median Length of Hospital Stay | 21.0 days (10.0-41.0) | 20.0 days (9.0 – 38.0) | N/A |
| Survival until Hospital Discharge | 128/4009 (3.2%) | 91/3995 (2.3%) | 1.48 (1.10 – 2.00) |
| Survival at 3 months | 121/4009 (3.0%) | 86/3991 (2.2%) | 1.47 (1.08 – 2.00) |
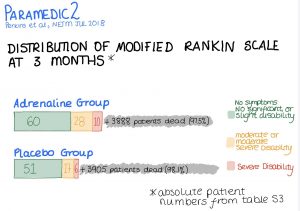
| Favourable Neurological Outcome on Discharge | 87/4007 (2.2%) | 74/3994 (1.9%) | 1.19 (0.85 – 1.68) |
| Favourable Neurological Outcome at 3 months | 82/3986 (2.1%) | 63/3979 (1.6%) | 1.39 (0.97 – 2.01) |
| Favourable neurological outcome defined as a score of 3 or under on modified Rankin scale | |||
Infographic by @WhistlingDixie4, embedded here with permission. This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Authors’ Conclusions
- The pre-hospital use of epinephrine in patients with OOHCA resulted in a significantly higher rate of survival at 30 days when compared to a placebo
- There was no significant difference between groups with regards to a favourable neurological outcome
- However, more survivors in the epinephrine group had more severe neurological impairment.
Strengths
- Pragmatic design with large numbers of randomised patients to answer an important question with a limited evidence base
- This trial provides the largest set of randomised data to date addressing this topic
- Good internal validity due to rigorous methodology
- Excellent strategy for concealment of random sequence to prevent selection bias
- Well balanced baseline variables suggests there is no allocation bias
- Successful blinding of trial participants and patients, which prevents performance bias and detection bias
- Very few patients (n=7) not included in primary analysis, reducing possible attrition bias
- Good external validity (generalisability)
- Multiple ambulance services enrolled patients across varying parts of the UK
- Balanced baseline characteristics
- Analysis adjusted for measured covariates
- Outcomes clinical and patient centred
- During design of trial 280 lay people were consulted surrounding personal views on the topic
- Of these, 95% prioritized long-term survival with a favorable neurological outcome over short-term (hours – days) survival
- Uses the NNT for other important interventions in the “chain of survival” such as bystander CPR (NNT 15) and early defibrillation (NNT 5) when contextualising the results of this trial
- Included Bayesian analysis for alternative interpretation
Weaknesses
- Although national guidelines exist for post cardiac arrest care, hospital care was at discretion of treating teams
- This is unlikely to have introduced performance bias, given the rigorous blinding of group allocation
- Although statistically significant, the fragility index with respect to 30 day mortality is only 6
- As there were 7 patients randomised but not included in this primary outcome, it is possible that the statistical significance could be lost had these 7 been included in the analysis
- The exclusion of those with early ROSC reduces the generalisability to that cohort
- The discussion surrounding neurological outcome is a very important one and well presented however:
- The study’s primary outcome was mortality, not neurological outcome so the power to detect meaningful differences in this important secondary outcome will be low
- There was significant loss to follow-up for the secondary outcomes
- 20 (18.2%) in the placebo group and 29 (18.2%) in the epinephrine group were lost at 3 months
- This may lead to attrition bias if there are differences between the patients lost from one group compared to the other group
- This weakens the strength of the conclusion we can draw from the secondary outcome results
- For the survivors, there was a difference in the rate of unfavourable neurological outcome at hospital discharge (epinephrine 31.0% vs placebo 17.8%) and at 3 months (16.3% vs 14.9%), so what is the best time frame to study neurological outcomes
- Is this observed difference due to patients that survived with unfavourable outcomes (possibly due to epinephrine administration) dying in the community after they leave hospital?
- However, these observed differences were not statistically significant due to the small numbers of patients surviving and this could just be variation by chance
- Overall survival rate was lower than anticipated leading to a review of the power calculation by the DMC
- Sample size was kept the same, which provided 90% power to detect a relative risk of 1.6 (compared to initial plan of 1.25)
- This corresponded to an Absolute Risk Reduction in mortality of 1.1%, which was still acceptable compared to the internationally agreed, clinically meaningful ARR of 1%
- Not generalizable to in-hospital setting due to dramatically different times to CPR and any drugs given
- Not strictly weaknesses, but the study produces a lot of unanswered questions:
- Cardiac arrest is the final common pathway of multiple different physiological events so would some subgroups of arrest causes benefit more than others from epinephrine?
- As survival to hospital was more likely in the Epinephrine group, could this be important as newer treatments evolve, such as Extracorporeal Membrane Oxygenation assisted CPR, as a bridge to these treatments?
- Would different dosing regimes alter outcomes?
- It has been suggested that a trial of [IV access + epinephrine] vs [no IV access + no epinephrine] would be important, given the possible impact from delays whilst gaining IV or IO access
The Bottom Line
- Naturally, one’s practice should be guided by Resuscitation Council guidelines
- However, this trial suggests epinephrine improves 30 day-mortality but it may be at the cost of worse neurological outcome
- This is a very pragmatic, large and well-delivered trial that poses a lot more clinical and moral questions
- Furthermore, this study highlights poor survival rates for OOHCA and the fact that more needs to be done to allow better access to defibrillators and early CPR
- This is something we can all help, with apps such as GoodSAM and events such as “Restart a Heart Day”
External Links
- https://www.resus.org.uk/resuscitation-guidelines/adult-advanced-life-support/
- Hagihara A et al. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307(11):1161-8. DOI: 10.1001/jama.2012.294
- Testing Epinephrine for Out-of-Hospital Cardiac Arrest. Callaway C.W. and Donnino M.W. New England Journal of Medicine, July 2018. DOI: 10.1056/NEJMe1808255
- Restart a Heart Day – https://www.resus.org.uk/events/rsah/
- https://www.goodsamapp.org
Metadata
Author: George Walker
Date: 27 July 2018
Peer-review editor: Duncan Chambler





WHICH WAS THE SITE OF EPINEPHRINE ADMINISTRATION?
Intravenous or Intraosseous