Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis

Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis
de-Madaria. NEJM 2022;387:989-1000. DOI 10.1056/NEJMoa2202884
Clinical Question
- In adults with acute pancreatitis does aggressive fluid resuscitation compared to moderate resuscitation result in a reduction in the development of moderately severe or severe pancreatitis?
Background
- Acute Pancreatitis (AP) has a high associated mortality and morbidity
- Rates of ICU admission with AP and associated multiorgan dysfunction have been reported to be as high as 25%, with up to 20% of these patients dying
- Emerging evidence suggests that across multiple pathologies a conservative fluid strategy is not harmful
- Despite limited high quality evidence and suggestion of harm in a systematic review and meta analysis the practice of aggressive fluid resuscitation is widely carried out
- The WATERFALL study was carried out to try and provide some high quality evidence to address this practice and its potential harms
Design
- Multi-centre, open-label, parallel-group, randomized, controlled, superiority trial
- Unblinded to both patients and investigators
- Patients enrolled from those presenting to ED < 24 hours from pain onset and < 8 hours from diagnosis
- Regular physical and biochemical assessments at 3,12, 24,48 and 72 hours conducted
- Clinicians were able to provide further fluid boluses, cease fluid or change rate based on certain goal directed points at these time points
- Computer based central randomization
- Stratified by centre, presence of SIRS and presence of hypovolaemia
- 1:1 ratio
- Independent DSMB consisting of clinical pharmacist, cardiologist and gastroenterologist
- 3 a-priori stopping rules were: (1) p values showing no difference at first and second interim analyses (< 0.0002 and then < 0.012 respectively), (2) clear harm demonstrated in one group and (3) a slow recruitment rate
- Power calculation:
- 744 patients required (alpha 0.05, 80% power) to detect a 10% point difference in development of moderately severe or severe AP from 35 to 25%
- Incidence of 35% based on Spanish multi-centre cohort study
Setting
- 18 centres in 4 countries (Spain, India, Italy, Mexico)
- May 2020 to September 2021
Population
- Inclusion:
- > 18 years old
- AP as defined by Revised Atlanta Criteria (2 or more of: typical abdominal pain, lipase or amylase 3x ULN or imaging evidence of AP)
- Exclusion:
- Those with moderately severe or severe AP at baseline (shock, respiratory failure, and renal failure)
- Cardiac Failure: NYHA II to IV
- Electrolyte abnormalities (Na < 135, > 145; K > 5)
- Chronic pancreatitis
- Chronic renal failure
- Decompensated chronic liver disease
- Severe comorbidity with life expectancy < 1 year
- 676 assessed —> 249 randomized
- 122 to aggressive group
- 127 to moderate group
- All those not randomized due to exclusion criteria or not providing consent
- Comparing baseline characteristics of intervention vs. control group
- Age: 56 vs 57
- Female: 55.7 vs 46.5%
- Gallstone AP: 65.6 vs 55.9%
- SIRS: 28.7% vs 22.8%
- Hypovolaemia: 52.5% vs 51.2%
- Median Urea: 32 vs 36 mg/dL
- Median Creatinine: 0.8 vs 0.8 mg/dL
- Median BISAP: 1 vs 1
- Median PAN-PROMISE: 31 vs 27
- Charlson Co-morbidity score: 2 vs 2
- Cumulative median volumes (L) of lactated ringers administered (Table S3)
- Aggressive vs Moderate
- 12 hrs: 3.4 vs 1.5
- 24 hrs: 5.4 vs 3.3
- 48 hrs: 7.8 vs 5.5
Aggressive Fluid Regime
- 20 ml/kg bonus then 3ml/kg infusion for 48 hours if oral feeding tolerated for > 8 hours
- Further 20ml/kg bolus allowed if UO < 0.5 mls/kg/hr or SBP < 90 mmHg during assessments at 12-72 hours
Moderate Fluid Resuscitation
- 10 ml/kg bolus only if hypovolaemic followed by infusion 1.5 ml/kg/hr for 20 hours if oral intake tolerated for > 8 hours
- Further 10 ml/kg boluses allowed as per intervention group above
Management common to both groups
- Lactated Ringers was fluid used
- Fluid rate able to be reduced or stopped if evidence of overload
- Clear definitions of volume state provided
- Oral feeding at 12 hours if PAN-PROMISE < 5
Outcome
- Study was stopped by DSMB as significantly worse results with respect to safety outcomes in the aggressive-resuscitation group, which were not balanced by any trend toward improved outcomes
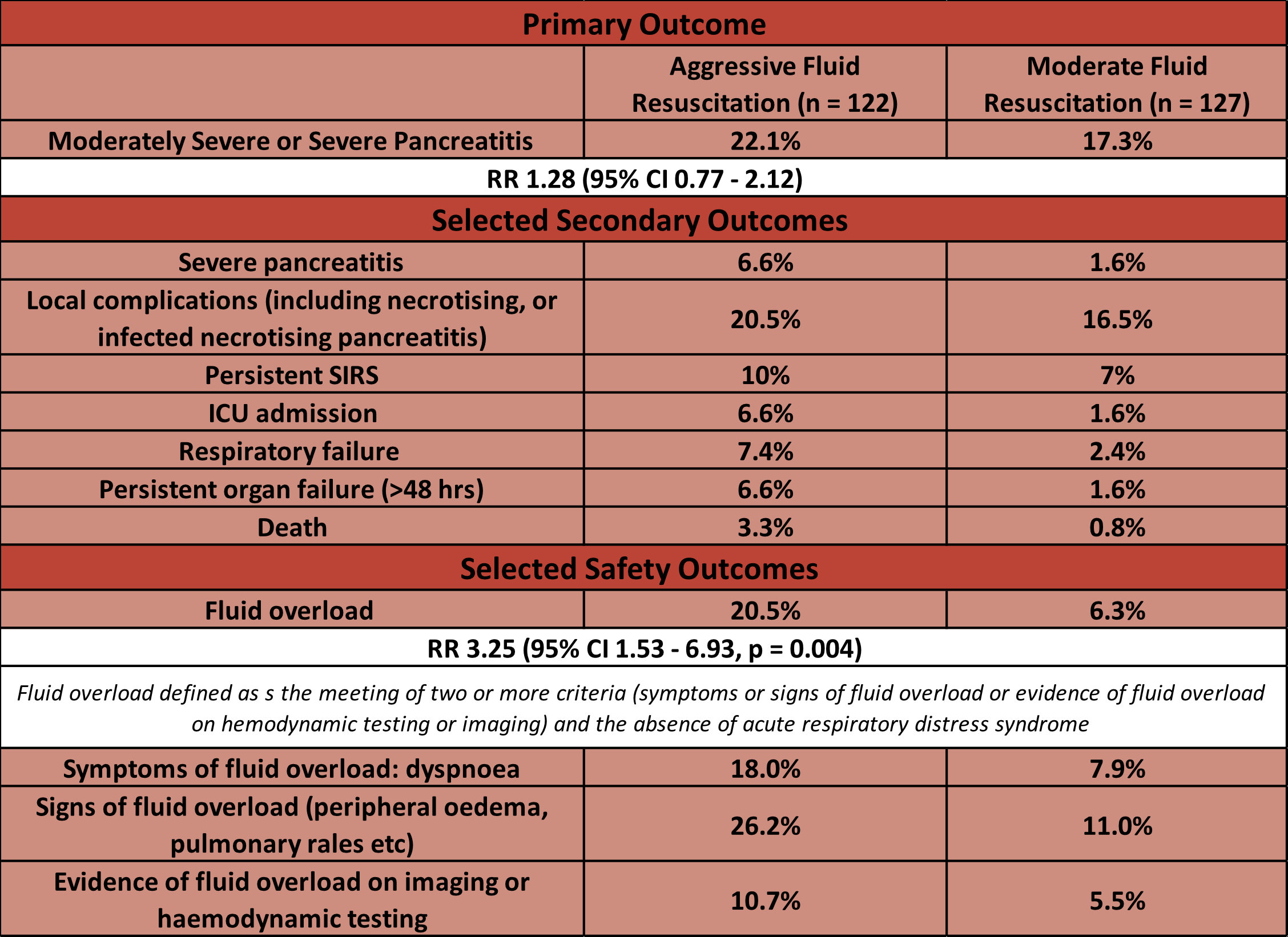
- Primary outcome: occurrence of Moderately Severe or Severe AP
- 22.1 % (aggressive) vs 17.3% (moderate)
- RR 1.28 (95% CI 0.77 – 2.12)
- Secondary and safety outcomes:
- Comparing aggressive fluid resuscitation vs. moderate fluid resuscitation
- No significant difference (although all trend towards favouring moderate resuscitation) in:
- Severe pancreatitis
- Local Complications
- ICU admission
- Persistent Organ Failure
- Persistent SIRS
- Death
- Significantly greater in aggressive group
- Fluid overload
- No significant difference (although all trend towards favouring moderate resuscitation) in:
- No difference from overall analysis in pre-specified subgroups (baseline SIRS and hypovolaemia)
Authors’ Conclusions
- In AP, aggressive fluid resuscitation as compared with moderate fluid resuscitation for the treatment of acute pancreatitis, led to a higher risk of volume overload and did not show benefit in disease-specific outcomes
Strengths
- Randomized, multi-centre
- No loss to follow up
- Intention to treat analysis
- Relatively balanced baseline characteristics
- Clear protocol for fluid administration and pre-specified definitions standardises outcome assessment and improves internal validity
- The removal of clinical signs that could have significant inter-observer variability further improves reproducibility (e.g. third and fourth heart sounds and pleural effusions on CXR)
- Excellent focus on safety outcomes given potential harm shown by high volume fluid administration
Weaknesses
- 86% of patients were from Spain
- Unblinded
- Given trend towards benefit of moderate fluid resuscitation with respect to the primary outcome, early cessation may have prevented study reaching a significant threshold
- Fairly significant exclusion criteria – the patients randomised have mild pancreatitis without significant co-morbidity (low Charlson Co-morbidity scores in each group)
- Uncertain if fluids prior to randomization or if any other fluids aside from Lactated Ringer’s were included in fluid totals administered
- Patient safety is always a priority but 20% in aggressive group compared to 8% in moderate group discontinued intervention (Figure S1) – this may bias results towards null
The Bottom Line
- Although this study excludes those patients with anything other than mild AP, this cohort of patients may still be seen on the ward by critical care teams. This trial provides robust evidence that high volumes of fluid administration lead to fluid overload
- This didn’t have significant clinical sequelae in this trial (intubation, RRT, death), however positive fluid balances have been associated with worse morbidity and mortality in the critically ill so is still an important factor we should consider
External Links
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 20th September 2022
Peer-review editor: David Slessor
Picture by: Pexels / Rifqi Ramadhan




Hi.
Thanks for great reviews.
Please note hyper/hyponatraemia exclusion criteria outside range 135-145 in the supplementary appendix, not 145-155 in the summary above.
Thanks David for spotting – I will update.