TRUST
TRUST: A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin
@EddCarlton, Heart 2015, Online First Feb 17, doi:10.1136/heartjnl-2014-307288
Clinical Question
- In patients with suspected acute coronary syndrome (ACS) does a novel accelerated diagnostic protocol (ADP) identify low risk patients suitable for discharge after a single high-sensitivity troponin T (hs-cTnT)?
Design
- Prospective observational study
- Consecutive patients
- Blinding of medical staff to initial hs-cTnT result
- Emergency physician staff completed ADP
Setting
- Single emergency department, UK
- July 2012 – August 2013
Population
- Inclusion criteria
- Adult patients with ≥5 minutes of chest pain suggestive of ACS
- Attending physician determined inpatient evaluation was required
- Exclusion criteria included
- ECG changes
- STEMI, LBBB not known to be old, ST depression ≥1mm, T wave inversion consistent with presence of ischaemia, arrhythmias
- Age ≥80
- Atypical symptoms in absence of chest discomfort
- Clear non-ACS cause for chest pain found at presentation
- Renal failure requiring dialysis
964 patients recruited, 4 patients lost to follow up
Test of Interest
- TRUST (Triage Rule-out Using high-Sensitivity Troponin) Accelerated Diagnostic Protocol
- Considered low risk if all of the following
- Modified-Goldman score of 0 or 1
- 1 point for each variable present
- Typical new onset chest pain at rest
- Pain the same as previous MI
- Pain not relieved by GTN within 15 minutes
- Pain lasts >60 minutes
- Pain occurring with increasing frequency
- BP <100mmHg
- Acute shortness of breath
- Pain within 6 weeks of MI/revascularisation
- Non-ischaemic ECG
- Hs-cTnT <14ng/L at presentation
- Modified-Goldman score of 0 or 1
- Blood taken for hs-cTnT at a median of 2 hours 55 minutes after chest pain onset
- Considered low risk if all of the following
Gold Standard Investigation
- Risk assessment by ED staff using modified Goldman risk score
- Hs-cTnT at 6 hours after presentation
- Follow-up at least 6 months after attendance by independent review of hospital records, GP records and national clinical records search
5th generation ELECSYS hs-cTnT assay used for both test of interest and gold standard
For all patients
- Myocardial infarction (MI) diagnosed according to Third Universal Definition of MI
- Adjudication of primary outcome carried out by two local cardiologists who were blinded to m-Goldman score but not to serial hs-cTnT results
Outcome
- Primary outcome: diagnostic accuracy of ADP for fatal or non-fatal MI within 30 days (including index visit)
- ADP identified 39.8% of patients at low risk of primary outcome
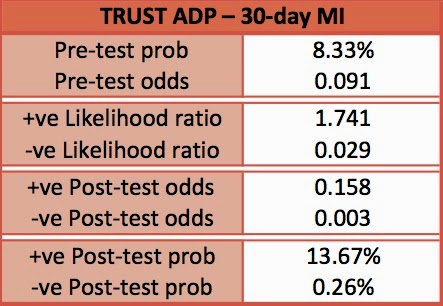
- 8.3% of all patients had MI within 30 days
- ADP 98.8% (95% CI 92.5-99.9) sensitive for ruling out MI
- Single patient had false negative test
- 78 year old female had initial hs-cTnT of 13 that rose to 20. Patient managed medically and had no further complications
- Secondary outcomes:
- Diagnostic accuracy of ADP for Major adverse cardiac events (MACE) within 30 days
- Death due to ischaemic heart disease, cardiac arrest, urgent revascularisation, cardiogenic shock, ventricular arrhythmia, high-degree atrioventricular block needing intervention, acute myocardial infarction
- 10.1% of all patients had MACE within 30 days
- ADP 99% (95% C.I. 93.7-99.9) sensitive for ruling out MACE
- Diagnostic accuracy of using a single initial hs-cTnT of <5ng/L (did not include risk assessment i.e. modified Goldman score)
- For fatal or non-fatal MI within 30 days
- Sensitivity 100% (95% C.I. 94.3-100)
- For MACE
- Sensitivity 96.8% (90.6-99.2)
- 3 false negatives, all aged in their 40’s and required urgent revascularisation. Two patients with severe left anterior descending artery disease and one patient with severe right coronary artery disease
- For fatal or non-fatal MI within 30 days
- Diagnostic accuracy of ADP for Major adverse cardiac events (MACE) within 30 days
Authors’ Conclusions
- The accelerated diagnostic protocol has the potential to allow early discharge in 40% of patients with suspected ACS
Strengths
- Prospective
- Appropriate inclusion + exclusion criteria leading to appropriate study population
- Blinding of medical staff to initial hs-cTnT result
- All patients received gold standard test
- Minimal loss to follow up
Weaknesses
- Single centre
- Exclusion of patients >80 years limits external validity
- With moderate numbers of patients included, the 95% confidence intervals for the primary outcome are wider than we would hope for (95% CI 92.5-99.9)
Of note, patients had initial hs-cTnT at nearly 3 hours after chest pain onset (shortly after they arrived in the Emergency Department). The ADP may therefore not apply to patients who present earlier
The Bottom Line
- The TRUST ADP is a very promising tool that may enable clinicians to rapidly exclude ACS in low risk patients. As the authors state, a multi-centre validation trial is required to confirm these findings. Ideally this would compare the TRUST ADP with the MACS rule
Conflict of Interest
- The lead author is a friend who I have previously worked with
External Links
- [article abstract] A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin
- [Further Reading] The MACS Rule
- [Further Reading + Podcasts] St. Emelyns: Everything you need to know about Troponins
- [Further Reading] Pre + Post Test Odds and Probabilities
Metadata
Summary author: @davidslessor
Summary date: 19th Feb 2015
Peer-review editor:@DuncanChambler




What is the rush . Better to do two tests one hour apart .Plus repeat ECG .