IRONMAN
Intravenous iron or placebo for anaemia in intensive care: the IRONMAN multicentre randomized blinded trial
; First online September 30th, 2016. doi:10.1007/s00134-016-4465-6
Clinical Question
- In critically ill patients who are anaemic, does early administration of intravenous iron compared to placebo reduce the requirement for blood transfusion?
Design
- Randomised, placebo-controlled trial
- Double-blinded design with opaque sleeve covering study drug
- Adequacy of blinding assessed with sub-study
- Online software produced 1:1 randomisation sequence stratified by study centre
- Sealed, opaque, numbered envelopes maintained allocation concealment
- Power calculation was based upon observational data suggesting a mean of 4 red blood cell (RBC) units transfused with a standard deviation (SD) or 2 units
- 140 patients allowed a detection of a clinically meaningful 1 unit difference with a false negative chance of 20% (80% Power) and a false positive chance of 5% (alpha significiance 0.05)
- The primary outcome was not normally distributed and therefore the analysis was changed from parametric testing (as published a priori) to non-parametric testing with reporting of median rather than mean units transfused
Setting
- 4 Intensive Care Units in Perth, Western Australia
- June 2013 to June 2015
Population
- Inclusion: age 18 or older; within 48 hours of ICU admision; anticipated to require ICU care beyond next day; anaemic with Hb < 100 g/l within preceeding 24 hours
- Exclusion: suspected or confirmed severe sepsis; ferritin > 1,200 ng/ml; transferrin saturation greater than 50%
- 140 patients randomised
Intervention
- Intravenous (IV) Iron
- 500 mg ferric carboxymaltose
- Made in 100 ml 0.9% NaCl
- Given as two consecutive 50 ml syringes
Control
- Placebo
- 100 ml 0.9% NaCl
- Given as two consecutive 50 ml syringes
Common management of both groups
- On day 4, patients still in ICU were assessed for a repeat dose of iron or placebo as per randomisation group
- This was given if they fulfilled the inclusion / exclusion criteria
- Repeat dosing assessment was made daily until four doses had been give, the patient died, or the patient was discharged from ICU
- Patient management was otherwise at the blinded treating physicians’ discretion
- This included the administration of red blood cell (RBC) transfusions (primary outcome)
- Open-label IV iron or erythropoiesis-stimulating drugs were considered protocol violations
Outcome
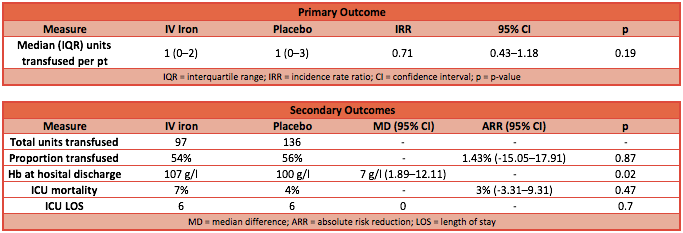
- Primary outcome: there was no significant difference in the median number of RBC units transfused between the IV iron group and the placebo group (from randomisation until hospital discharge)
- IV iron group: median RBC units was 1 [IQR 0-2]
- Placebo group: median RBC units was 1 [IQR 0-3]
- P-value = 0.53
- Incident rate ratio (IRR): 0.71 (95% CI 0.43 to 1.18; p-value 0.19)
- Secondary outcome:
- Median Hb was significantly higher in the IV iron group at discharge from hospital: 107 g/l vs 100 g/l (p = 0.02)
- Median lengths of stay were not significantly different
- Mortality rates were not significantly different
- Adverse event rates were not significantly different
Authors’ Conclusions
- In anaemic patients who are admitted to ICU, IV iron did not result in a reduction in red blood cell transfusion compared to placebo, however they did leave hospital with a higher haemoglobin level.
Strengths
- Multi-centre randomised trial
- Robust blinding of patient and treating clinicians
- Adequate randomisation and concealment of allocation
- Minimal cross-over
Weaknesses
- Despite attempting to represent all ICU patients, a large proportion were cardiothoracic surgical (35%) or trauma (32%) patients
- Medical patients only made up 14%
- This reduces the generalisability of the study to other units and populations
- The mean units transfused was 1.9, which is much less than the expected 4.0
- This reduces the effective power of the study
- It is possible that a false negative conclusion (type 2 error) has been drawn
- The distribution of the primary outcome was not as anticipated, leading to a post-hoc change in the analysis
- Whilst the statistical methods are appropriate, this potentially reduces the strength of the internal validity and the study’s conclusion
The Bottom Line
- This study is inconclusive due to the impact of the unanticipated low rate of transfusion
- The point estimate favours IV iron, but the confidence intervals are wide
- Further studies are needed before the widespread use of IV iron can be rejected or implemented
External Links
- [article] Intravenous iron or placebo for anaemia in intensive care: the IRONMAN multicentre randomized blinded trial
- [further reading] Anaemia by LITFL
- [further reading] Managing Anaemia in Critical Care by Prof Walsh
Metadata
Summary author: Duncan Chambler
Summary date: 8 December 2016
Peer-review editor: Celia Bradford




Pingback: IRONMAN – The Bottom Line #FOAMed #FOAMcc – Critical Care Northampton
Nice page! Thank you!