SuDDICU

Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial
SuDDICU Investigators. JAMA 2022; doi:10.1001/jama.2022.17927
Clinical Question
- In mechanically ventilated adults, does the addition of selective decontamination of the digestive tract (SDD) compared to standard care reduce all-cause hospital mortality within 90 days?
Background
- SDD has been around for nearly 40 years
- SDD is the application of non-absorbable antimicrobials to the oropharynx and GI tract in combination with a short course of IV antibiotics to target gram negative bacteria, Staph Aureus and yeasts
- Multiple systematic reviews have reported statistically significant reductions in both hospital mortality and hospital acquired pneumonia
- Despite this, international uptake is low with concerns about generalisability of previous RCTs and around development of antibiotic resistance
- A large RCT in 2018 found no increased rates of multi-drug resistance with SDD
- SuDDICU was designed to help address this uncertainty
Design
- Cross-over, cluster randomised controlled trial:
- ICUs were stratified by size and randomly assigned by computer generated program to either use SDD in the first 12-month period, or continue standard care
- ICUs then crossed over to the alternate choice for the second 12-month period
- Concomitant observational ecological assessment:
- Non-inferiority design
- Data on all new organisms in blood and non-blood cultures, positive C.Difficile test results and any antibiotic resistance from cultures was collected
- Data collected for 1 week per month of each three-month ecology periods (pre-trial, inter-period, post-trial and last 3 months of each 12 month trial period)
- All patients in the ICU were included in ecology data collection, except those ventilated in the SDD intervention group
- Time flow of intervention and ecology periods:
- 3-month pre-trial period
- Ecological assessment only
- Intervention period 1 (12 months)
- Both intervention and ecological assessment
- 3-month inter-period gap
- Ecological Assessment only
- Intervention period 2 (12 months)
- Both intervention and ecological assessment
- 3-month post-trial period
- Ecological assessment only
- 3-month pre-trial period
- Waiver of individual consent up to hospital discharge was obtained as intervention was ICU, or patients received no intervention (control and ecological groups)
- Appropriate trial registration and ethical approvals
- Power Calculation
- SDD: 6000 patients from 20 ICUs provided an absolute 4.2% reduction from a baseline mortality rate of 29%
- 80% power, alpha of 0.05
- Ecological Assessment: 40-50 ICUs recruiting 110-150 patients per period had 80% power to reject a non-inferiority margin of 2% for antibiotic resistance (based on an incidence of 10% for resistance)
- SDD: 6000 patients from 20 ICUs provided an absolute 4.2% reduction from a baseline mortality rate of 29%
Setting
- 17 hospitals (19 ICUs) in Australia
- May 2017 to November 2021
Population
- Inclusion:
- Mechanical ventilation via ETT on admission to ICU, or requirement mechanical ventilation during ICU admission
- Predicted to remain intubated for > 48 hours
- Patients could be rescreened if ventilated for > 48 hours
- Exclusion:
- Enrolled in a trial that would interact with intervention
- Known allergy to drugs used
- Known or suspected pregnancy
- Not expected to survive 12 hours
- Site inclusion and exclusion criteria published in Table 1 of protocol summary and statistical analysis plan
- 14851 enrolled
- 5982 in intervention study
- 2791 SDD
- 3191 Standard Care
- 8599 in ecological assessment
- 5982 in intervention study
- Comparing baseline characteristics of SDD vs. standard care group
- Age: 58 vs 59
- Male: 64 vs 63%
- ICU admission source
- ED: 40 vs 37%
- Emergency Surgery: 20 vs 22%
- Elective Surgery: 6 vs 7%
- Time from ICU admission to enrolment: 16.1 vs 3.7 hrs
- APACHE III: 68 vs 73
- Comorbidities
- Diabetes: 22 vs 23%
- Systemic Steroids: 12 vs 13%
- Immunosuppression: 8 vs 9%
- Prior Treatments
- IV antibiotics: 75 vs 68%
- Oral Chlorhexidine: 28 vs 17%
Intervention
- SDD consisted of:
- 6 hourly application of 0.5g of paste containing 10 mg of colistin, 10 mg of tobramycin, and 125 000 IU of nystatin applied to the buccal mucosa and oropharynx
- 6 hourly administration of 10 mL of gastric suspension containing 100 mg of colistin, 80 mg of tobramycin, and 2 × 106 IU of nystatin to the upper gastrointestinal tract via a gastric or postpyloric tube
- 4-day course of an intravenous SDD-compliant antibiotic (eg, a third-generation cephalosporin or ciprofloxacin)
- Not required if already treated with antibiotics with activity against gram-negative bacteria during the first 4 days after enrolment
- Administered ASAP after ICU admission and enrolment
- Continued to cessation of ventilation via ETT or day 90
Control
- Standard Care
- Oral Chlorhexidine allowed
Management common to both groups
- All other treatment, including prophylactic and therapeutic antibiotic use, at discretion of treating clinicians
Outcome
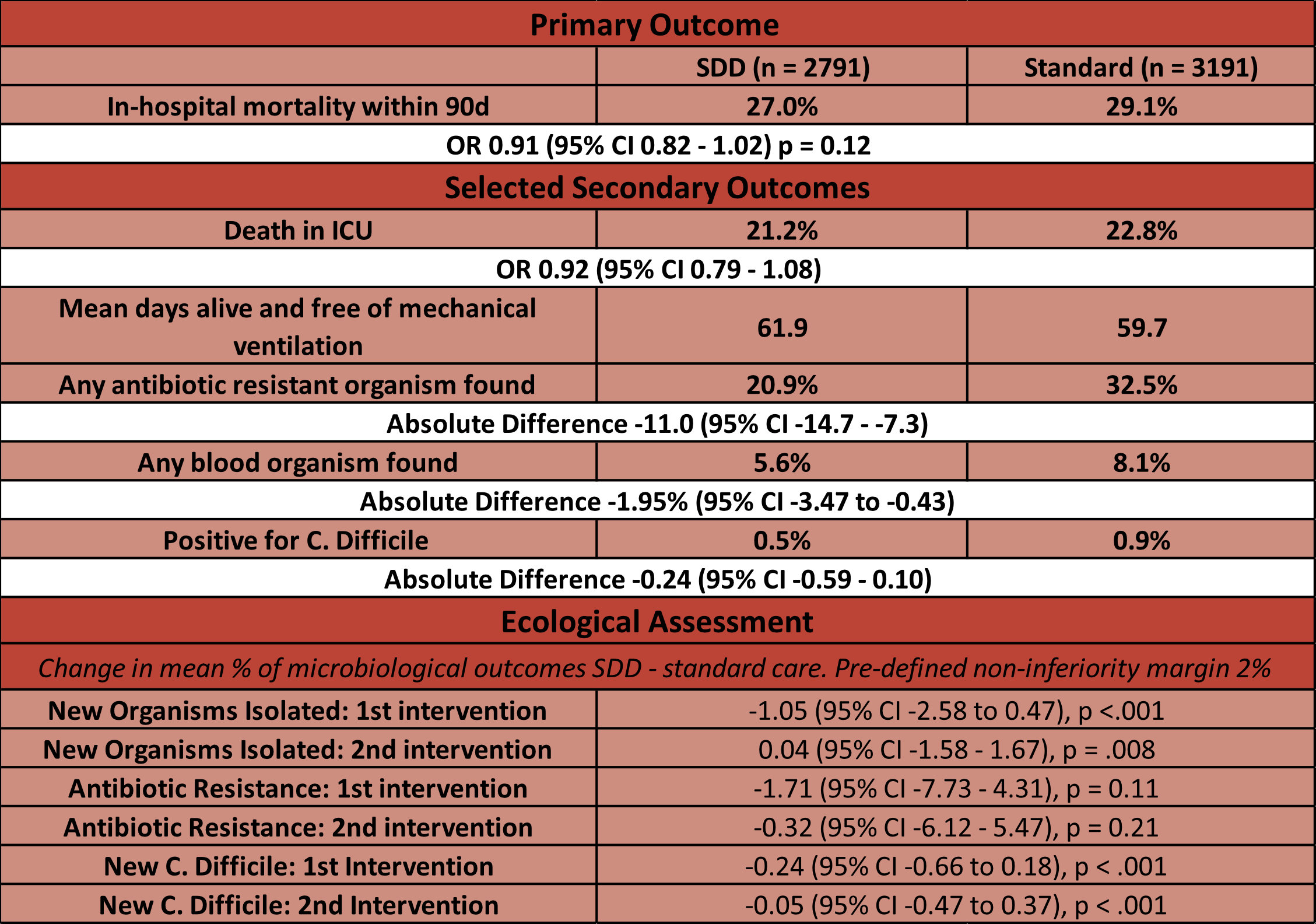
- Primary outcome: In-hospital 90-day mortality – non significant
- 27.0% (SDD) vs 29.1% (Standard Care)
- OR 0.91 (95% CI 0.82 – 1.02), p = 0.12
- Secondary outcomes:
- Comparing SDD vs. standard care group
- No significant difference in
- Death in ICU: 21.2% vs 22.8%
- Days alive and free from mechanical ventilation: 62 vs 60
- C. Difficile positive test: 0.5% vs 0.9%
- Adverse medication events: 0 vs 0
- Significantly less in SDD group
- Any antibiotic resistant organism found: 20.9% vs 32.5%
- Any blood organism found: 5.6% vs 8.1%
- No significant difference in
- Subgroups
- No significant effect in any of the pre-defined subgroups
- Ecology
- SDD non-inferior to standard care with respect to change in proportion of newly positive blood cultures, or C. Difficile infection but not for cultures of antibiotic resistant organisms
Authors’ Conclusions
- The use of SDD did not reduce in-hospital mortality, however the confidence interval does not exclude a clinically important benefit
Strengths
- High quality RCT with very strong internal validity
- Excellent and highly detailed ecological analysis is crucially important given prior concerns
- Significant exposure to SDD within ICUs is important for ecological analysis – approximately ~65000 doses of both suspension and paste given
- Cluster, crossover design allows adequate evaluation of unit microbiology and ecology; individual patient randomisation may not have allowed this
- Pre-published protocol and statistical analysis plan
- Balanced baseline characteristics
- No loss to follow up
Weaknesses
- Single country may limit external validity, especially in countries where antimicrobial usage and resistance patterns are different
- Canadian centres have had slower recruitment, and their data will be published separately – this will hopefully improve external validity with the inclusion of data from an additional country
- 1203 protocol violations (Supplement 4 eTable 14) in SDD group
- ~10% for SDD paste not being administered for one full day, ~13% SDD antibiotics not being administered for one full day, ~10% neither paste not antibiotics administered within 6 hours of enrolment
- A detailed log for non-administration is supplied
- Approximately ~50% of all patients in standard care arm received SDD compliant IV antibiotics – this may bias result towards null (Supplement 4 eFigure 5b)
- Post hoc analyses adjusting for antibiotics and chlorhexidine didn’t alter overall findings
- Low rates of antimicrobial resistance found in all cultures (~10%, eTable 11) and relatively short periods of ecological data collection may limit power to determine SDD non-inferiority with regards to ecological outcomes
- Uncertainty persists as to what SDD regime is best
- The subgroup looking at use of IV or no IV antibiotics in the simultaneously published meta analysis seems to show a benefit only with IV antibiotic usage
- Powered to detect a 4.2% reduction in mortality – this may have been overly optimistic
The Bottom Line
- Although this trial did not show a statistically significant benefit of SDD with respect to in hospital 90-day mortality, the associated Bayesian meta-analysis suggests that SDD has a 99.3% posterior probability of lower hospital mortality (Moderate certainty of evidence)
- This mortality benefit does seem hard to ignore; however SDD implementation would require multi-speciality buy-in and would need safe systems for the analysis for the enormous amount of ecological data that would need to be collected and monitored to ensure safety with regards to any developing antimicrobial resistance patterns
External Links
- Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial
- further reading Systematic Review and Meta-Analysis
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 31st October 2022
Peer-review editor: @davidslessor
Picture by: Gerd Altmann / Pixabay



