SUP-ICU
Pantoprazole in Patients at Risk for Gastrointestinal Bleeding in the ICU
Krag M et al. NEJM October 2018 DOI: 10.1056/NEJMoa1714919
Clinical Question
- In ICU patients at risk of gastrointestinal (GI) bleeding does the use of prophylactic proton-pump inhibitor (PPI) compared to placebo reduce mortality at 90 days?
Background
- Critically ill patients are at risk stress-related upper GI bleeding with its associated mortality and morbidity
- The adverse events associated with PPI use include nosocomial pneumonia, C. difficile enteritis and myocardial ischaemia
Design
- Multicentre, stratified, placebo-controlled, blinded (pts, clinicians, investigators and statisticians)
- Computer-generated, block randomisation, stratified according to trial site
- 1:1 assignment to intervention or placebo
- 90% power to detect an absolute difference of 5% in 90 day mortality between groups
- Assuming baseline mortality of 25% at 90 days
- 3350 patients would be needed
Setting
- 33 ICUs in 6 countries (Denmark, Finland, Netherlands, Norway, Switzerland and UK)
- January 2016 to October 2017
Population
- Inclusion:
- 18 years or older
- non-elective ICU admission
- at least 1 risk factor for GI bleeding
- Shock
- Renal replacement therapy
- Invasive mechanical ventilation > 24 hours
- Coagulopathy
- Ongoing treatment with anticoagulants
- History of coagulopathy
- History of chronic liver disease
- Exclusion:
- Contraindications to PPI
- Ongoing treatment with PPI and/or H2-antagonists
- GI bleeding during current hospital admission
- Confirmed diagnosis of peptic ulcer disease
- Organ transplant during current hospital admission
- Withdrawal of active treatment
- Fertile woman with positive urine human chorionic gonadotropin (hCG) or plasma-hCG
- 3298 pts recruited
- Baseline characteristics were well matched except for a higher incidence of chronic lung disease and coagulopathy in intervention group, and a higher proportion of pts post emergency surgery in the control group
- Median age 67, 64% men, median SOFA score 9
Intervention
- 40mg Pantoprazole
- Single, once a day bolus suspended in 10mls 0.9% saline
Control
- Placebo
- Single, once a day bolus suspended in 10mls 0.9% saline
Management common to both groups
- Medication continued from randomisation until ICU discharge or death, for a maximum of 90 days
- All other interventions were chosen at the discretion of the clinician
Outcome
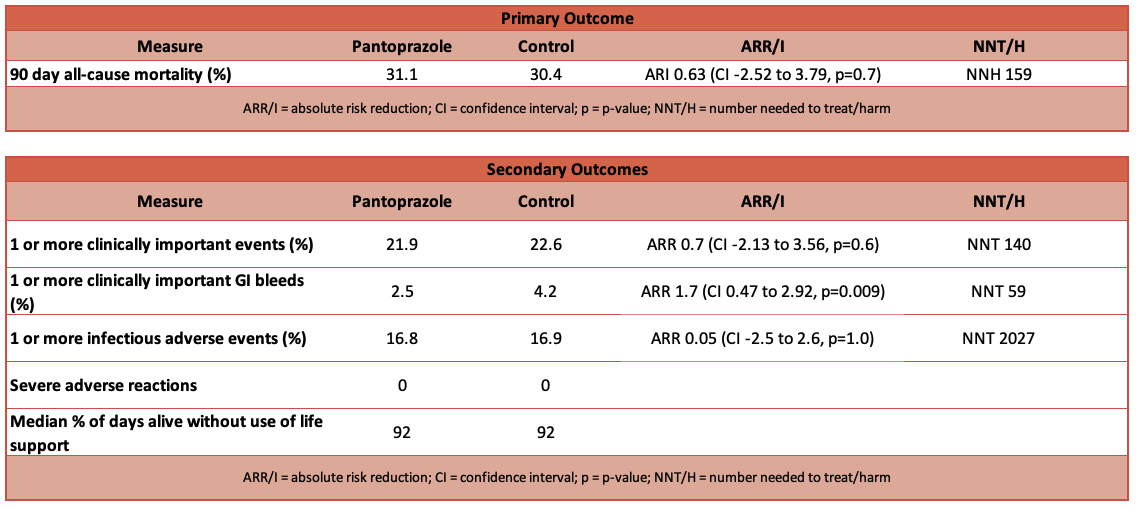
- Primary outcome: 90-day all cause mortality
- 31.1% (intervention) vs 30.4% (control)
- Secondary outcome:
- Proportion of patients with 1 or more of the following clinically important adverse events – GI bleeding, pneumonia, C difficile infection, acute myocardial ischaemia
- 21.9% (intervention) vs 22.6% (control)
- Clinically important GI bleeding
- Defined as overt GI bleeding and at least 1 of the following: spontaneous drop of systolic, mean or diastolic BP of 20mmHg or more, start of vasopressor or 20% increase in vasopressor dose, decrease in Hb of at least 2g/dl, transfusion of 2u packed RBCs
- 2.5% (intervention) vs 4.2% (control)
- Defined as overt GI bleeding and at least 1 of the following: spontaneous drop of systolic, mean or diastolic BP of 20mmHg or more, start of vasopressor or 20% increase in vasopressor dose, decrease in Hb of at least 2g/dl, transfusion of 2u packed RBCs
- Infectious adverse events in the ICU
- Proportion of pts with 1 or more episodes of pneumonia (according to modified CDC criteria) or C difficile infection (treatment with antibiotics for suspected or proven C difficile infection)
- 16.8% (intervention) vs 16.9% (control)
- Proportion of pts with 1 or more episodes of pneumonia (according to modified CDC criteria) or C difficile infection (treatment with antibiotics for suspected or proven C difficile infection)
- Serious adverse reactions in the ICU
- none in both groups
- Days alive without the use of life support (median)
- 92 (intervention) vs 92 (control)
- Proportion of patients with 1 or more of the following clinically important adverse events – GI bleeding, pneumonia, C difficile infection, acute myocardial ischaemia
Authors’ Conclusions
- In adult patients admitted to the ICU for an acute condition and were at risk for gastrointestinal bleeding, there was no significant differences between pantoprazole and placebo with regard to either 90-day mortality or the number of patients with a composite outcome of four clinically important events.
Strengths
- Large, multicentre with appropriate blinding strategy
- Relevant clinical question
Weaknesses
- Composite secondary outcome was not a well-used, established one
- No P values provided for secondary outcomes because of the lack of adjustments for multiple comparisons (accepting limitations of interpreting secondary outcomes)
- Very broad inclusion criteria and definitions (both of ‘high risk’ and outcomes)
- Powered to detect 5% mortality improvement which is perhaps unrealistic
The Bottom Line
- Although no mortality benefit was demonstrated, the incidence of clinically important GI bleeds (secondary outcome and hence the usual caveats apply), was lower in the pantoprazole group
- This trial is about starting PPI so does not change my current practice of stopping acid-suppression medication in patients established on enteral feed except in high risk groups (high dose corticosteroids, high dose vasopressors etc)
External Links
- [article] Pantoprazole in Patients at Risk for Gastrointestinal Bleeding in the ICU
- [further reading] Accompanying editorial
- [further reading] PulmCrit- SUP-ICU: Is pantoprazole the elixir of life? Should it be?
Metadata
Summary author: Adrian Wong
Summary date: 1 November 2018
Peer-review editor: David Slessor



