ATACAS Asp

Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) Trial
Myles et al. NEJM 2016; 374: 728-737 doi: 10.1056/NEJMoa1507688
Clinical Question
- In adults undergoing elective coronary artery graft surgery does the administration of aspirin prior to surgery compared to a placebo result in increased mortality or thrombotic complications?
Background
- Aspirin is given to patients for primary or secondary prevention of cardiac events, stroke and death
- A side-effect of antiplatelet therapy is bleeding, which is of concern in the peri-operative setting
- Historically, advice has been that aspirin should be stopped before major cardiac surgery to reduce this bleeding risk, despite the possible increase in the thrombotic risk
- A meta-analysis of smaller trials suggests that continuation of aspirin throughout the peri-operative period may reduce the risk of death or thrombosis at the cost of increased bleeding, blood product requirement and surgical re-exploration
Design
- Double blinded randomised control trial
- Registered with Australia and New Zealand Clinical Trials Registry (ACTRN12605000557639)
- 2 x 2 factorial design, whereby patients assigned to receive 100mg aspirin or a placebo and tranexamic acid (TXA) or a placebo
- Tranexamic arm was subsequently published and has been critiqued by TBL: ATACAS-TXA
- Written informed consent provided and trial approved at each site
- Computer generated randomisation, stratified by site with permuted blocks allowed, assigned in a 1:1 ratio
- Power calculations based on detection of a clinically significant absolute risk difference of 3% (10% versus 7%) in primary outcome (death or thrombotic events) resulted in aim to recruit 4600 patients
- Protocol amended in July 2015:
- Enrollment rate lower than anticipated primarily because patients continued to take aspirin in line with their local recommendations
- Primary outcome event rate was higher (at 19.6%) than expected so steering group decided to discontinue aspirin arm and deemed with 1880 already enrolled, this would have 90% power to detect a 30% relative risk reduction among those continuing to take aspirin before surgery
- Interim analysis planned at 2300 and 3450 patients, and a safety analysis undertaken after 824 patients to ensure no excessive risk of bleeding
- Sites that recruited >30 patients independently audited
- No discrepancies found
- Funded by Australian National Health and Medical Research Council, the Australian and New Zealand College of Anaesthetists and the National Institute of Health Research
- No conflicts of interests disclosed
- Drugs and placebo provided by Bayer without further involvement
Setting
- 22 centres across 6 countries
- 2006 – 2013
Population
- Inclusion: Adult patients undergoing elective coronary artery surgery (on and off pump), whom were at increased risk of major complications
- Age > 70
- LV impairment
- Concomitant valvular or aortic surgery
- Repeat surgery and medical co-morbidities (COPD, CKD, Obesity, PVD and pulmonary hypertension)
- Eligible if not taking aspirin or aspirin had been ceased for at least 4 days prior to surgery
- Exclusion criteria related to bleeding risk or previous thromboembolic disease, as well as urgent surgery and surgeon preference for anti-coagulation
- Similar patient demographics, medical and peri-operative characteristics at enrollment (Aspirin vs. Placebo)
- Age 66.5 vs. 66.2 yrs
- Male sex 83.3% vs. 81.5%
- EuroSCORE 4.1% vs. 4.1% (measure of operative risk)
- Diabetes 33.1% vs. 34.9%
- Hypertension 80.9% vs. 80.2%
- Heart Failure 13.0% vs. 12.6%
- MI within 90 days 7.2% vs. 7.9%
- Previous cardiac surgery 1.6% vs. 1.3%
- Cross clamp time (min) 67 vs. 66
- Duration of surgery (hrs.) 3.8 vs. 3.8
- Postoperative aspirin within 24 hours 78.4% vs. 76.0%
- 5784 patients eligible, of which 2127 randomised, and 2100 analysed for primary outcome (1.3% loss to follow-up)
Intervention
- Aspirin
- 100mg enteric coated tablets were given 1-2 hours before surgery
Control
- Placebo
- Identical placebo tablets were given 1-2 hours before surgery
Management common to both
- 50% in each arm also received TXA
- Warfarin and clopidogrel ceased for 1 week prior to surgery
- Remaining anticoagulants managed at discretion of local unit
- Both groups received standard surgical and other peri-operative care
- There was no limit to use of postoperative aspirin or other antiplatelets
- Guidelines on heparin / protamine use, management of excessive bleeding and blood transfusions provided
- Patients assessed daily in hospital and had phone follow up at 30 days
- ECGs taken pre-operatively and on days 1,2 and 3 and on hospital discharge, biochemistry obtained 12-24 hours post surgery and 48 – 72 hours post surgery for troponin or CK-MB depending on local availability
- 75% underwent primary CABG of which 97% were on pump
- The average number of grafts was 3, with 90% receiving at least one internal mammary graft.
- The definition of a non fatal MI was:
- Typical rise and fall of cardiac biomarkers with at least one of:
- Ischaemic symptoms
- Development of pathological q waves
- Ischaemic ECG changes
- Findings at autopsy of an acute MI
- Given difficulty in detecting ischaemic chest pain post operatively a non-Q wave MI was diagnosed using any of:
- Troponin I > 10ng/ml, Troponin T > 4.0 at any time or CK-MB > 5x upper limit of normal > 12 hours post op
- Typical rise and fall of cardiac biomarkers with at least one of:
Outcome
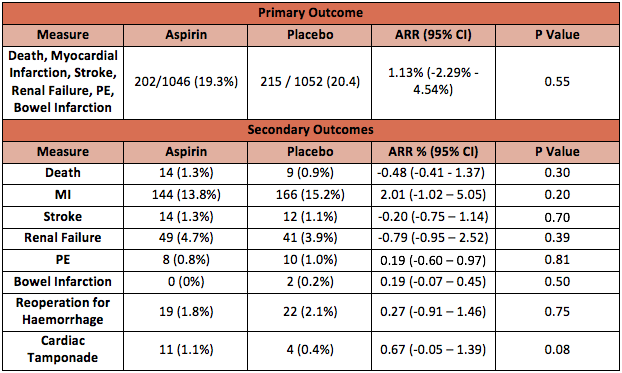
- Primary outcome: a composite of death and thrombotic events (non fatal MI, CVA, PE, renal failure or bowel infarction) in the first 30 days post procedure
- No clinical or statistical significant difference was found
- Aspirin group: 19.3%
- Placebo group: 20.4%
- Relative risk (as published): 0.94 (95% CI 0.80 to 1.12; P = 0.55)
- Absolute risk reduction (re-calculated): 1.12% (95% CI -2.29% to 4.54%; P = 0.55)
- Secondary outcomes:
- There was no statistically significant difference in any of the individual outcomes of the composite primary outcome (data presented in table below)
- Mediastinal drainage and numbers receiving any blood product transfusion within 24 hours were similar,
- Although not statistically different there were more platelet transfusions on day 1 in the aspirin group
- 15% vs. 13.1%
- RR 1.14 (P = 0.23)
- ARR -1.89% (95% CI -1.08 to 4.86%)
- More red cells were transfused from day 2 to discharge in the aspirin group
- 18.1 % vs. 16.1%
- RR 1.19 (P = 0.08)
- ARR -2.96%. (95% CI -0.29 to 6.21)
- Although not statistically different there were more platelet transfusions on day 1 in the aspirin group
- ICU stay, hospital stay and duration of mechanical ventilation were not statistically different between the groups
Authors’ Conclusions
- There is no evidence that pre-operative aspirin administration resulted in a lower risk of death or thrombotic complications, or a higher risk of haemorrhage.
Strengths
- Large multi-centre RCT allows for external validity in this cohort of patients
- Reasonable follow up period given duration of action of single aspirin dose
- Excellent similarities between patient groups at baseline
- Analysed on an intention to treat basis
- Good quality control methods used throughout
- Appropriate statistical analysis
Weaknesses
- The 2×2 design meant that half of patients receiving aspirin also received TXA
- The sensitivity of the interaction analysis may not be adequate to draw firm conclusions
- With higher numbers of platelets transfused on day 1 and more red cells transfused from day 2 to discharge there is a possibility this may have been statistically significant without the concomitant use of TXA
- The study stated high-risk patients were included, however the average EuroSCORE was under 5%, which fits more with medium and low risk patients
- This reduces the studies external validity for higher risk patients
- Recruitment was ceased early due to higher event rates than anticipated, but the composite end-point included sub-acute (biomarker elevation only) MIs which could be argued as not equivalent in weight against death
- The study may be underpowered to detect an important difference in clinically significant MIs or death
- Large numbers of patients were not enrolled despite being eligible
- 3657 patients did not consent of which 1143 had a surgeon that did not want to enroll and 1594 gave no reason
- Could the enteric-coated aspirin have variable absorption and thus result in different clinical effects in different subjects
- A study looking at EC coated aspiring in diabetics showed a high proportion of patients with type 2 diabetes had “aspirin resistance”
- Short follow up – 30 days phone call only
- The study aim (and title) was to compare stopping vs continuing aspirin, however the design insisted on all patients stopping aspirin and then being given a single dose of aspirin or placebo prior to surgery (and presumably all patients were given aspirin after surgery) – this method hasn’t really investigated the theory
The Bottom Line
- This was a large, multi centre RCT that claims to show that a single dose of aspirin administered pre-operatively for patients undergoing coronary artery surgery does not result in increased risk of bleeding or a lower risk of death or thrombotic complications
- However the methodological issues raised above and the possible difference between a single pre-operate aspirin vs regular daily aspirin mean this study is unlikely to change practice without further work
External Links
- [article] Stopping vs Continuing Aspirin before Coronary Artery Surgery
- [correspondence] Aspirin before Coronary Artery Surgery – Letters to the Editor
- [further reading] Aspirin and Coronary Artery Surgery: a systematic review and meta-analysis by Hastings et al.
- [further reading] Enteric Coating and aspirin non-responsiveness in patients with Type 2 Diabetes Mellitus by Bhatt et al.
Metadata
Summary author: George Walker
Summary date: 13 September 2017
Peer-review editor: Duncan Chambler



