REMAP-CAP
 Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial
Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial
REMAP-CAP Investigators. J
Clinical Question
- In patients with severe COVID-19, does intravenous hydrocortisone (either as a fixed dose or restricted to when shock is clinically evident) compared with standard care, improve 21-day organ support free days?
- The study is part of the wider REMAP-CAP trial which aims to determine the best treatment strategies for patients with severe pneumonia (both in pandemic and non pandemic settings)
Background
- COVID-19 has resulted in 13,710 critical care admissions in the UK (to 7th September 2020) of which ~39% have died
- Recent trials investigating the use of steroids in COVID-19 include RECOVERY and the CoDEX trial
- A meta-analysis, of 7 randomised trials including REMAP-CAP, published in JAMA on the same date as this trial, found that corticosteroid administration was associated with a lower 28 day mortality
Design
- Adaptive platform trial
- International, multicenter, open-label
- Patient’s randomised to multiple interventions within multiple domains
- A domain is a common therapeutic intervention, in this case the use (or no use) of hydrocortisone
- Other domains included anti-viral, targeted immune modulation, immunoglobulin and therapeutic anticoagulation
- Patient’s can be randomised to multiple domains
- e.g. hydrocortisone and antiviral medication
- Randomised via computer software in balanced manner
- Pre-specified secondary outcomes
- Staff not blinded to treatments
- International Trial Steering Committee were blinded
- Sample size calculated based on trial simulations and with 90% power to determine if either group was superior to no hydrocortisone
- If OR 1.75 – 500 patients
- If OR 1.5 – 1000 patients
- Bayesian statistics used
- Please see “Bayesian Statistics” review for more detailed explanation
- Primary model adjusted for site, age, sex and time period (2 week blocks), and pre-specified interactions in the model between various domains
- Primary analysis conducted on all patients who met inclusion criteria, not just those randomised within corticosteroid domain
- Re-analysis of primary outcome then repeated in patients enrolled in corticosteroid domain
- Internal statistical triggers for conclusion of trial and dissemination of results were pre-existing
- Designed so that an OR >1 shows benefit
- Written or verbal informed consent from all participants or surrogates
- Relevant ethical approval at all sites
- Registered on clinicaltrials.gov
Setting
- 121 sites in multiple countries
- March 9th – June 17th 2020
- Due to reporting of RECOVERY results steering committee decided to cease recruitment to the corticosteroid domain due to lack of equipoise
- No data had been reviewed prior to this decision
- Due to reporting of RECOVERY results steering committee decided to cease recruitment to the corticosteroid domain due to lack of equipoise
Population
- Inclusion:
- Platform Inclusion (into REMAP-CAP):
- Adult patient admitted to hospital with suspected or proven COVID-19
- Corticosteroid Domain Inclusion:
- Severe disease state, defined by receiving respiratory (HFNC > 30L and FiO2 > 0.4, NIV or invasive ventilation) or cardiovascular support (any vasopressor or inotrope) in an intensive care unit (ICU)
- Platform Inclusion (into REMAP-CAP):
- Exclusion:
- Known hypersensitivity
- Intention to prescribe systemic corticosteroids for a reason unrelated to CAP / COVID-19 (e.g. chronic steroid use)
- Admission to ICU > 36 hours ago
- Prior randomisation into a trial evaluating corticosteroids
- Treating clinician believes not in patient’s best interest
- 1165 screened –> 614 enrolled in REMAP-CAP Severe COVID arm
- 403 enrolled into the corticosteroid domain
- 143 fixed dose, 152 shock dose and 108 to no hydrocortisone
- 403 enrolled into the corticosteroid domain
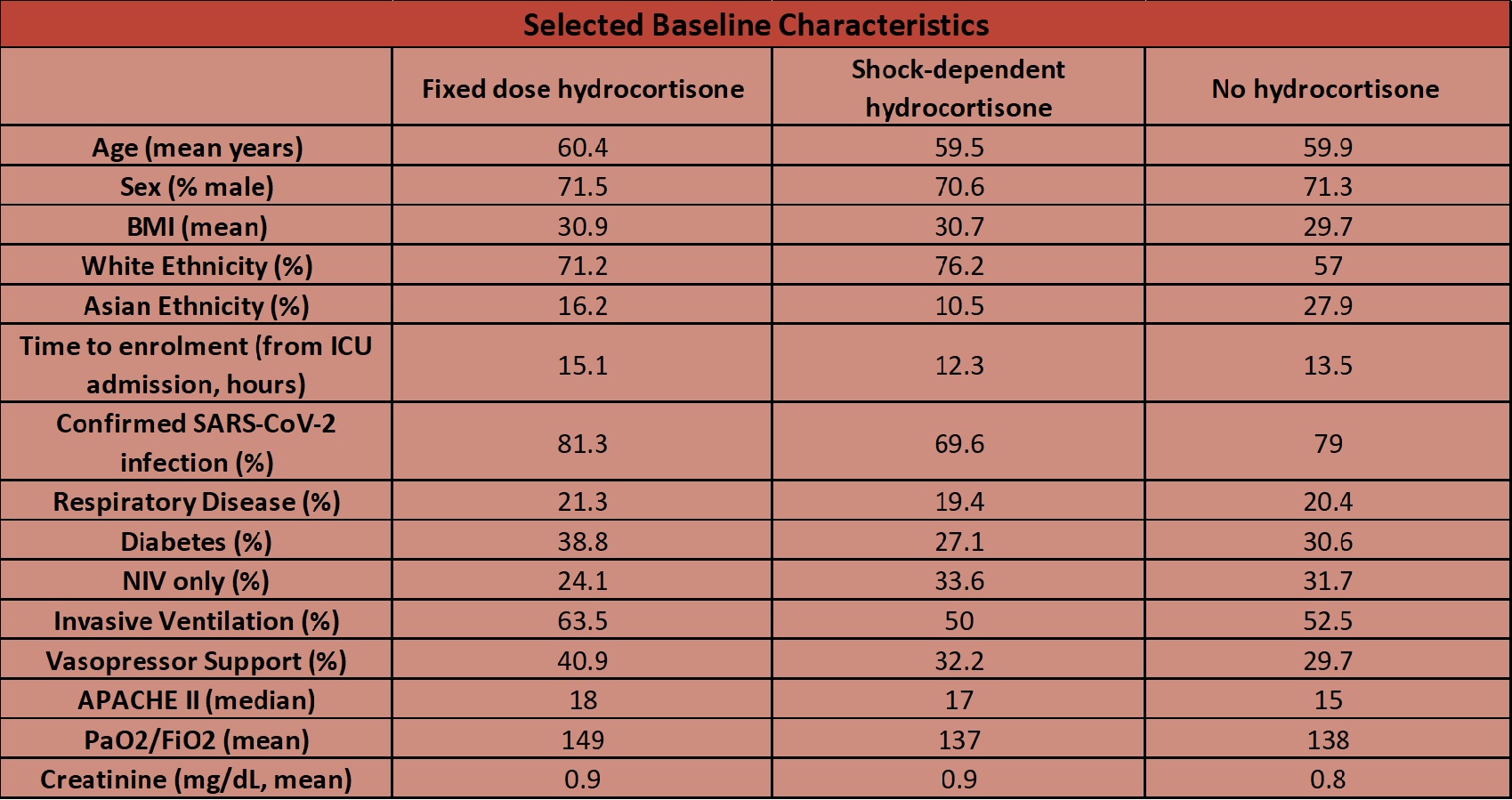
- Baseline demographics were fairly well balanced (as documented in table below)
Intervention
- Hydrocortisone administration
- Fixed dose: 50mg q6 hourly for 7 days (2 patients received 100mg q6 hourly)
- High dose hydrocortisone arm (100mg q 6 hourly) had been a treatment option but given only two patients received the high dose treatment prior to recruitment being stopped the two arms were combined into one “fixed dose hydrocortisone arm”
- These two treatments were “nested” together so this is amalgamation is permitted in an adaptive design
- n= 143, of whom 6 withdrew consent
- High dose hydrocortisone arm (100mg q 6 hourly) had been a treatment option but given only two patients received the high dose treatment prior to recruitment being stopped the two arms were combined into one “fixed dose hydrocortisone arm”
- Shock-dependent: 50mg q6 hourly whilst in shock (up to 28 days)
- Defined as the use of vasopressor due to COVID-19 (and not another cause such as sedation)
- n= 152, of whom 6 withdrew consent and a further 5 had outcome data that was not available
- 43% of patients received at least 1 dose of hydrocortisone with a median duration of 3 days
- Fixed dose: 50mg q6 hourly for 7 days (2 patients received 100mg q6 hourly)
Control
- No hydrocortisone
- n = 108, of whom 7 withdrew consent
Management common to both groups
- No ongoing management prescribed but patients could be randomised to other domains in the platform
- In all groups systemic corticosteroids could be given if a new clinical indication developed for which steroids are established treatment e.g. post-extubation stridor
Outcome
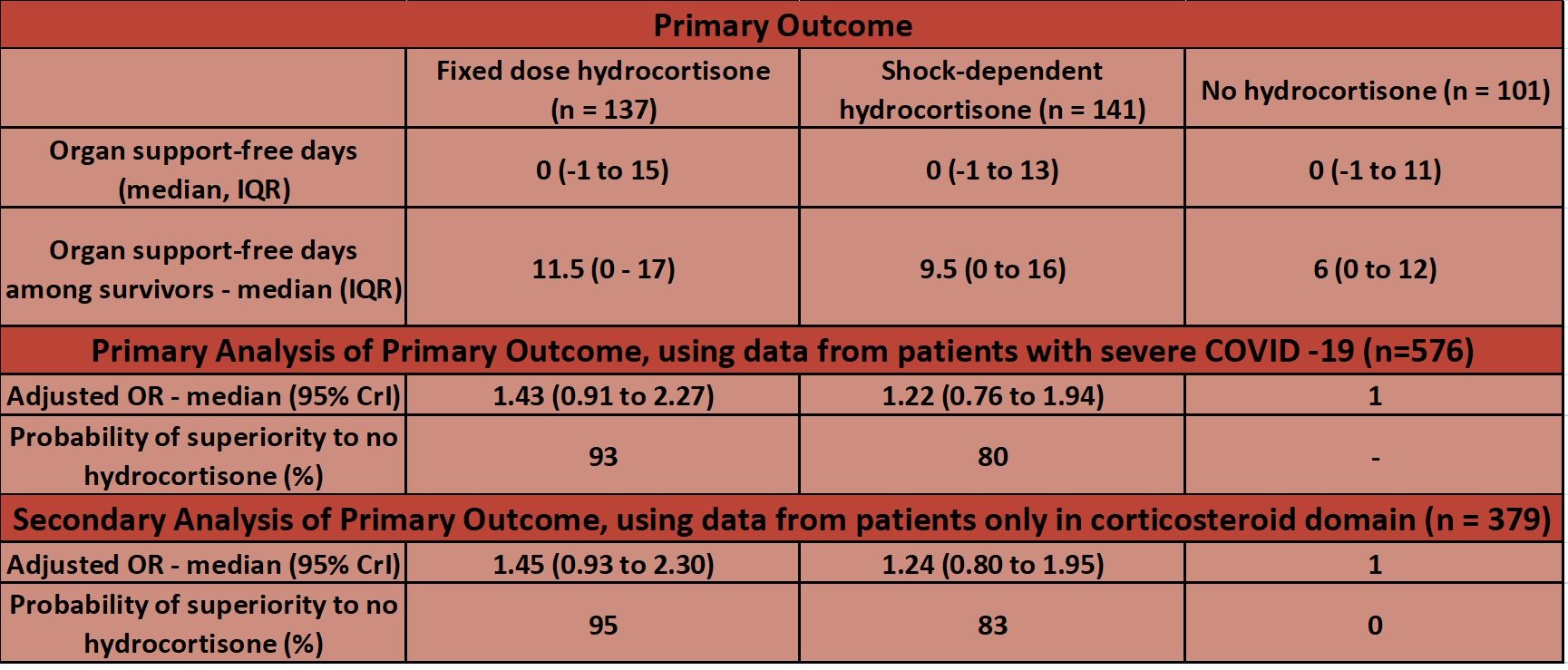
- Primary outcome:
- Respiratory and cardiovascular organ support-free days, up to day 21 – no significant difference
- Organ support defined using same criteria as study inclusion criteria
- Using data from all patients with severe COVID-19 the probability that hydrocortisone is beneficial if compared to no hydrocortisone when assessing number of organ free days is:
- 93% for a fixed regime
- 80% for a shock regime (note only 43% received hydrocortisone)
- Respiratory and cardiovascular organ support-free days, up to day 21 – no significant difference
- Secondary outcomes (comparing fixed dose vs. shock-dependent vs. no hydrocortisone; Odds ratio (OR) compared to no hydrocortisone group – fixed dose vs shock-dependent group)
- In hospital mortality
- 30% vs. 26% vs. 33%
- OR 1.03 (95% C.I. 0.53-1.95) vs. 1.10 (95% C.I. 0.58-2.11)
- % probability of superiority of hydrocortisone to no hydrocortisone (fixed dose vs. shock dependent in all patients with severe COVID-19)
- 54% vs. 62%
- In hospital mortality
- % probability of superiority of hydrocortisone to no hydrocortisone (fixed dose vs. shock dependent for patients solely in the corticosteroid domain)
- Time to death: 40% vs. 47%
- Respiratory organ support free days: 94% vs. 85%
- Cardiovascular organ support free days: 98% vs. 86%
- Length of ICU stay: 29% (fixed) vs. 14% (shock)
- Hospital length of stay: 43% vs. 31%
- WHO score at day 14: 87% vs. 55%
- Progression to invasive ventilation, ECMO or death (for those not intubated at baseline, n = 168): 99% vs. 70%
- Adverse events:
- Patient’s with serious adverse event:
- Fixed 3%, Shock 4%, None 1%
- Patient’s with serious adverse event:
Authors’ Conclusions
- Treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone (compared with no hydrocortisone) resulted in 93% and 80% probabilities of superiority with regard to the odds of improvement in organ support–free days within 21 days
- It is re-iterated that neither hydrocortisone groups met the pre-specified criteria for statistical superiority and thus definitive conclusions cannot be drawn
Strengths
- Multi-centre international, randomised trial with a pragmatic design
- The presentation of Bayesian results gives a “real-life” probability by which the treatment being tested may be beneficial:
- This appears easier to interpret for both clinicians and when discussing treatments with non clinicians
- The use of all patients, and not just those in the corticosteroid domain, enables the maximal amount of information available to be studied.
- This increases the accuracy of the adjustments made in the primary model (e.g. those for age, sex, time)
- The use of two analyses serves to confirm the stability of the results
- The prior based on information from ICNARC
Weaknesses
- 15% (n = 15) of the no hydrocortisone group did receive a systemic corticosteroid, of which the median duration was 2 days
- All groups were allowed to receive systemic corticosteroid therapy if a new clinical indication allowed (e.g. post extubation stridor)
- It could be debated as to whether this would affect outcomes – if steroids are favourable, then the administration to the no hydrocortisone group would only likely serve to reduce the probability by which corticosteroid administration is deemed to be beneficial
- Only 43% in the shock group received at least 1 dose of hydrocortisone
- The authors state that if corticosteroids are beneficial in COVID-19 (in ways other then mitigating shock) then this group maybe under-treated
- This may result in the 80% probability being underestimated
- The authors state that if corticosteroids are beneficial in COVID-19 (in ways other then mitigating shock) then this group maybe under-treated
- With regards to patient selection:
- of the 1165 patients assessed, 42 of these were excluded as the clinician intended to administer corticosteroids, and of the 614 who met the severe COVID-19 definition, 38 were excluded as the clinician intended to administer corticosteroids
- This may result in some selection bias as this is ~7% of all patients assessed
- of the 1165 patients assessed, 42 of these were excluded as the clinician intended to administer corticosteroids, and of the 614 who met the severe COVID-19 definition, 38 were excluded as the clinician intended to administer corticosteroids
- Groups not completely balanced at baseline – lower use of mechanical ventilation and vasopressors in the arm that received no corticosteroids
- Trial not blinded
- The authors make a very rational argument that blinding would have impacted significantly on the speed in which this trial was set up and able to enrol patients (which is important in a pandemic)
- 6% of patients withdrew consent or did not have outcome data available. There is concern of this introducing bias, particularly in an open label trial
- No low income countries randomised, and a large proportion (n = 419 in all domains) were randomised from the UK
- The cessation of the trial earlier than planned meant that the pre-defined statistical triggers were not reached and thus definitive statistical conclusions can not be drawn
- Given the trial was open label this was important to minimise (although highly unlikely) any protocol violations given the strongly positive effects shown in RECOVERY
- The authors acknowledge that no optimal strategy of corticosteroid use is shown by this study
- Prescott and Rice in their JAMA editorial highlight many further clinical questions that still need to be answered
The Bottom Line
- The fact that such a large, international trial has been so rapidly and rigorously conducted alongside a significant burden of clinical critical care work is remarkably impressive
- Although no statistical triggers were reached, the Bayesian approach gives a high probability of superiority of corticosteroid use (compared to no corticosteroid use) in organ support free days within 21 days. It also reported a large reduction in the progression to invasive ventilation, ECMO or death with the use of steroids
- Medicine is a balance of benefit and risk. With the high probability of benefit and low evidence of harm from this study, combined with the evidence from the RECOVERY trial and the WHO meta-analysis, I will continue to advocate for the use of corticosteroids in COVID-19
External Links
- ICNARC report on COVID-19 in Critical Care
- Life in the Fast Lane: Adaptive Trials
- St Emelyns: The “Roid” to Recovery
- REBEL-EM: The COVID-19 Ordinal Scale of Clinical Improvement
- Critical Care Reviews: REMAP-CAP Steroid Domain Results Podcast
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 9th September 2020
Peer-review editor:@davidslessor



