Steroids in Sepsis
Steroids in septic shock
We suggest against using IV hydrocortisone to treat septic shock patients if adequate fluid resuscitation and vasopressor therapy are able to restore hemodynamic stability. If this is not achievable, we suggest IV hydrocortisone at a dose of 200 mg per day (weak recommendation, low quality of evidence).
Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2016
Background
The hypothalamus secretes corticotrophin-releasing hormone (CRH), stimulating the release of adrenocorticotrophin hormone (ACTH) from the anterior pituitary. This results in cortisol secretion from the adrenal glands.
Normal serum cortisol levels are thought to range between 5 and 24 mcg/dL, with significant variability depending on the time of day. During physiological stress, hypotension, or severe infection, the Hypothalamic-pituitary-adrenal (HPA) axis is activated and diurnal variation is lost. Serum cortisol increases as a result, reaching levels as high as 40 to 50 mcg/dL. Suboptimal cortisol production during these periods has been termed ‘functional’ or ‘relative’ adrenal insufficiency. Whether serum cortisol response and exogenous administration in during episodes of functional insufficiency can predict and improve mortality respectively has been the focus of extensive debate for over 50 years.
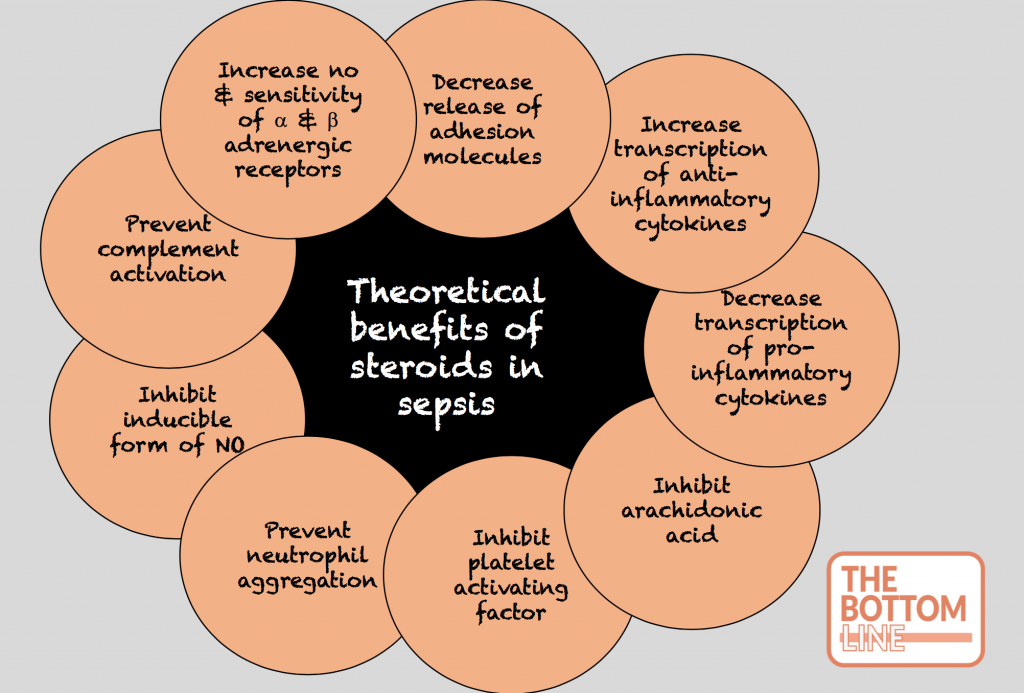
The theoretical benefit
The early clinical trials
1960s
- Bennett (1963) – the first prospective randomised, double-blind placebo-controlled studies of steroid administration (100 mg of oral hydrocortisone vs placebo) as an adjunctive strategy for patients with severe sepsis and septic shock
- Findings: No significant survival difference between the treatment and control groups
1970s
- Schumer (1976) – this study combined a prospective trial with a retrospective analysis contrasting two steroid regimens with placebo in adult surgical patients with septic shock
- Findings: Mortality rates of 11.6% and 9.3% were seen for patients treated with methylprednisolone (one or two doses of 30 mg/kg IV) and dexamethasone (one or two doses of 3 mg/kg IV), respectively, vs 38.4% for patients receiving placebo
- A concomitantly performed “retrospective” study showed similar results
- Based on this paper, it became standard practice in the late 1970s and early 1980s to administer high-dose corticosteroids at the onset of septic shock
- The study has a number of limitations based on our current understanding of sepsis and the evolution of study designs. These include: a single investigator enrolled all 500 patients (prospective and retrospective) in a single hospital over a 9-year period; sepsis defined as a “a septic history,” a “falling BP,” or positive blood cultures; a primary outcome of death attributable to septic shock was subjective, including “. . . if the patient succumbed immediately to the shock episode or had a continuing septic course with episodes of shock and then succumbed.”
1980’s
- 4 studies assessing mortality outcome in patients with septic shock were published. None showed any survival benefit with steroids in septic shock
- Sprung (1984) – 59 patients in a single centre received either methylprednisolone, 30 mg/kg IV, dexamethasone, 6 mg/kg IV, or placebo
- Findings: more rapid shock reversal but no survival benefit beyond 10 days of follow up
- Veterans Administration Trial (1987) – RCT with 233 patients randomised to methylprednisolone, 30 mg/kg followed by 5 mg/kg, or to placebo, administered within 3 h of diagnosis
- Findings: No difference in 14-day mortality or
complications was demonstrated
- Findings: No difference in 14-day mortality or
- Bone (1987) – RCT with 381 patients randomised to receive methylprednisolone, 30 mg/kg, or placebo
- Findings: higher mortality rate in the steroid group
- Luce (1988) – 87 patients in a single centre received either methylprednisolone, 30 mg/kg per dose, or placebo (mannitol) for a total of 4 doses every 6 h, following the presumptive diagnosis of septic shock
- Findings: no improvement in survival or prevalence of ARDS
- Sprung (1984) – 59 patients in a single centre received either methylprednisolone, 30 mg/kg IV, dexamethasone, 6 mg/kg IV, or placebo
- As a result of these pivotal trials, steroid use in septic shock began to dwindle in the late 1980’s and 1990’s. Supra-physiological doses of steroids were recognised as having no benefit and considered potentially harmful
2000’s
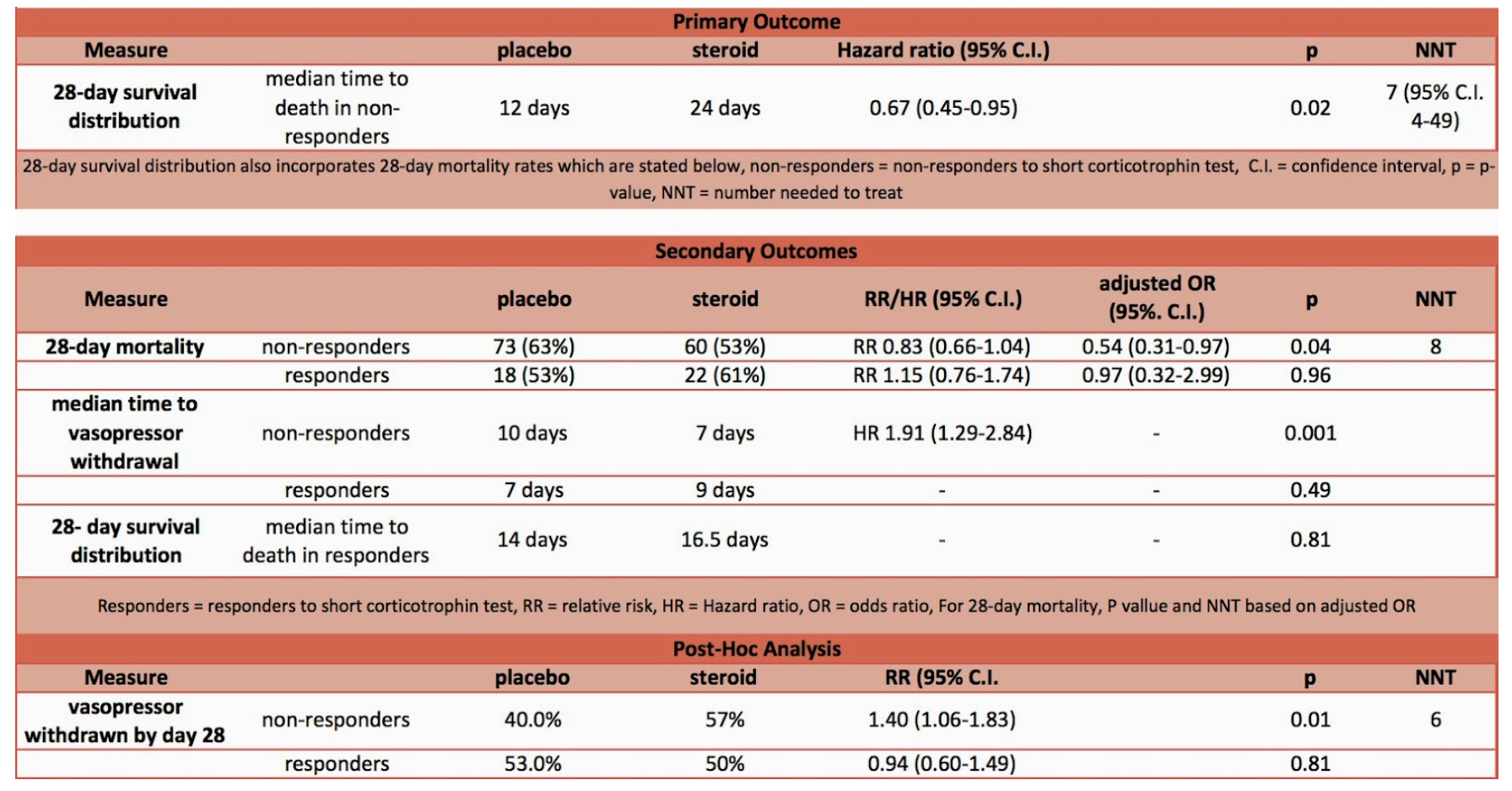
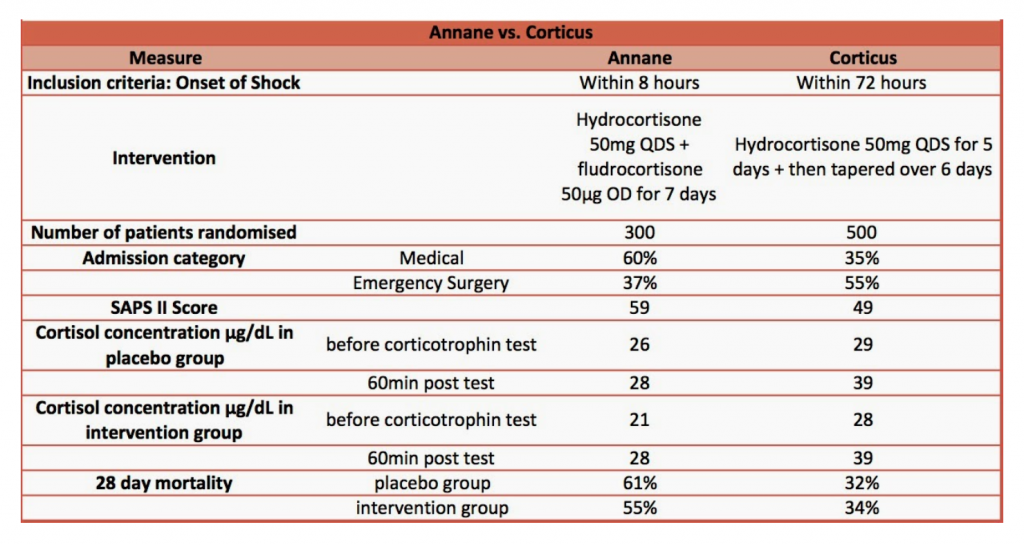
- Annane (2002) – a multicentre French study involving 19 ICU’s from 1995 to 1999 reignited the debate about the potential benefits of steroids in a select cohort of patients with septic shock. This study formed the basis for our use of exogenous glucocorticoids in septic shock for several years
- 300 patients were randomised within eight hours of the onset of septic shock to receive placebo or hydrocortisone (50 mg intravenously every six hours) plus fludrocortisone (50 mcg enterally once a day) for seven days
- Based upon a high dose (250 mcg) ACTH stimulation test, all patients were classified as having adequate adrenal reserve (maximum increase in serum cortisol of >9 mcg/dL = >250nmol/L) or inadequate adrenal reserve (maximum cortisol increase of 9 mcg/dL = <250nmol/L)
- Findings: Hydrocortisone administration was associated with decreased 28-day mortality (53% vs. 63 %), ICU mortality (58% vs. 70%), hospital mortality (61% vs. 72%) and shorter duration of vasopressor use in those patients with inadequate adrenal reserve (the ‘non responders’)
-
- The limitations of this study include:
- the statistical methods used to derive mortality
- an adjusted mortality analysis using a regression model derived from a prior study was used. This yielded lower P-values. The unadjusted P-value for 28 day mortality in the non-responders is 0.11
- 24% of patients received etomidate (inhibits adrenal corticosteroid synthesis)
- use of fludrocortisone, a potential confounder
- timing to antibiotics was delayed, compared to current standards (mean 6hrs in placebo vs. 7.1hrs in steroid group)
- the statistical methods used to derive mortality
- The limitations of this study include:
- Oppert (2005) – 41 consecutive patients with with early hyperdynamic septic shock were randomised to receive either low-dose hydrocortisone (50-mg bolus followed by a continuous infusion of 0.18 mg/kg body of weight/hr) or matching placebo
- Findings: treatment with low-dose hydrocortisone accelerates shock reversal in early hyperdynamic septic shock. This was accompanied by reduced production of proinflammatory cytokines, suggesting both haemodynamic and immunomodulatory effects of steroid treatment
- CORTICUS (2008) – 499 patients with severe sepsis recruited from 52 participating ICUs
- Severe sepsis based on all of the following:
- clinical evidence of infection; systemic response to inflammation; shock (systolic BP < 90 mmHg despite 1 hour fluid resuscitation, or the need for vasopressors); organ dysfunction attributable to shock
- Hydrocortisone given as 50 mg in 6-hourly boluses for 5 days and then tapered over 6 days vs placebo
- 499 patients recruited. This study under recruited based on the power calculations and in addition, the actual mortality (~38%) was much lower than estimated mortality used for power calculation (50%)
- Findings: no difference in 28-day mortality in short corticotropin non-responders (i.e. the sub-group that were more likely to benefit from exogenous corticosteroids)
- 39.2% in hydrocortisone group vs. 36.1% in placebo group
P=0.69 - See TBL review of CORTICUS for more details
- 39.2% in hydrocortisone group vs. 36.1% in placebo group
- Severe sepsis based on all of the following:
2010-2018
- COIITSS (2010) – A multicenter (11 ICUs in France), RCT, involving 509 adults with septic shock and SOFA score of 8 or more
- Factorial (2×2) assessing the efficacy of intensive insulin therapy in patients whose septic shock was treated with hydrocortisone (50mg bolus every 6 hours for 7 days) and to assess, as a secondary objective, the benefit of fludrocortisone
- Compared with conventional insulin therapy, intensive insulin therapy did not improve in-hospital mortality among patients who were treated with hydrocortisone for septic shock. The addition of oral fludrocortisone did not result in a statistically significant improvement in in-hospital mortality
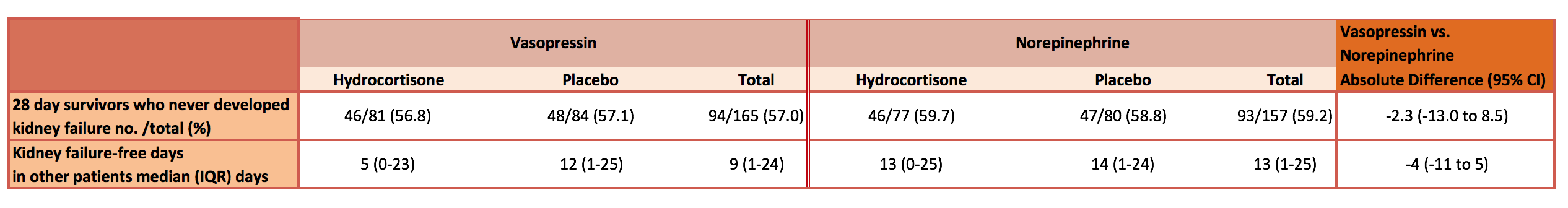
- VANISH Trial (2016) – 421 adult patients (≥16 years) who had sepsis (2 of 4 systemic inflammatory response criteria due to known or suspected infection) and who required vasopressors despite adequate intravenous fluid resuscitation
- Factorial (2×2) multicenter, double blind, RCT
- Study drug 1: Noradrenaline or Vasopressin
- Study drug 2: Hydrocortisone or placebo
- Early vasopressin maintains blood pressure and reduces the requirement for norepinephrine and renal replacement therapy. However, vasopressin does not reduce the number of renal replacement free days or mortality rate, and there was no clinical interaction with corticosteroids
- Factorial (2×2) multicenter, double blind, RCT
- Hydrocortisone, Vitamin C and Thiamine for the Treatment of Severe Sepsis and Septic Shock (2016) – Single centre, unblinded and retrospective observational study
- 47 patients with severe sepsis or septic shock and a procalcitonin (PCT) >2 ng/ml
- Vitamin C + Hydrocortisone + Thiamine in addition to standard treatment
- 1.5g QDS Vitamin C intravenously for 4 days or until ICU discharge
- Hydrocortisone 50mg QDS intravenously for 7 days or until ICU discharge followed by a taper over 3 days
- 200mg thiamine BD for 4 days or until ICU discharge
- Before and after study
- Primary outcome: Hospital mortality – 8.5% (4 of 47) in the treatment group compared to 40.4% (19 of 47) in the control group (p < 0.001)
- HYPRESS (2016) – randomised, double-blind, placebo-controlled, multicentre trial in 34 study sites in Germany
- Assessed whether hydrocortisone compared to placebo prevents the development of septic shock?
- Bolus of hydrocortisone iv 50mg followed by a 24 hr continuous infusion of 200mg for 5 days, 100mg for Day 6 & 7, 50mg on Day 8 & 9 and 25mg on Day 10 & 11. The continuous infusion was preferred to prevent unwanted undulation in blood glucose concentrations
- The placebo was lyophilized mannitol which was indistinguishable from the hydrocortisone (133mg mannitol – a tiny dose compared with therapeutic mannitol for raised ICP = 1g/kg)
- Administration of hydrocortisone did not prevent the development of shock in patients with severe sepsis
- Assessed whether hydrocortisone compared to placebo prevents the development of septic shock?
- Early initiation of low-dose hydrocortisone treatment for septic shock in adults: A randomized clinical trial (2017) – 118 patients with septic shock were randomised to receive hydrocortisone or 0.9% saline.
- The study medication (hydrocortisone and normal saline) was initiated simultaneously with vasopressors
- The early initiation of low-dose of hydrocortisone did not decrease the risk of mortality, and the length of stay in the ICU or hospital in adults with septic shock.
Systematic reviews and meta-analyses
Pre-CORTICUS
Annane (2004) – 16 RCTs or quasi-RCT’s, including 2063 patients were selected. For all trials, regardless of duration of treatment and dose, use of corticosteroids did not significantly affect mortality. With long courses of low doses of corticosteroids, however, mortality at 28 days and hospital morality was reduced.
Post-CORTICUS
Sligl (2009) – 8 studies (6 RCTs) involving a total of 1876 patients were selected. In patients with septic shock, corticosteroid therapy appears to be safe but does not reduce 28-day all-cause mortality rates. It does, however, significantly reduce the incidence of vasopressor-dependent shock.
Sherwin – (2012) – The Sepsis Sub-committee of the American Academy of Emergency Medicine Clinical Practice Committee identified seven relevant trials and concluded that low-dose corticosteroids may reverse shock faster; however, mortality is not improved.
Wang (2014) – 8 RCTs’ found low dose hydrocortisone therapy decreased shock at 7 and 28 days, with no change in mortality.
Cochrane review (2015) – included a total of 33 RCTs, accounting for 4268 hospitalised patients with sepsis. Three trials included children, and the remaining 30 trials included only adults. Corticosteroids were compared with placebo in all except five trials, in which they were compared with standard therapy alone. Low-quality evidence indicates that steroids reduce mortality among patients with sepsis. Moderate-quality evidence suggests that a long course of low-dose steroids reduced 28-day mortality without inducing major complications.
Gibbison (2017) – Complete data from 22 studies and partial data from 1 study were included. There was no clear evidence that any one corticosteroid drug or treatment regimen is more likely to be effective in reducing mortality or reducing the incidence of gastrointestinal bleeding or superinfection in septic shock. Hydrocortisone delivered as a bolus or as an infusion was more likely than placebo and methylprednisolone to result in shock reversal
The Bottom Line…so far
- Steroids do not reduce mortality in patients with sepsis based on the evidence to date. A RCT is required to further assess the efficacy and safety profile of combined steroid with thiamine and vitamin C
- Steroids may improve the rate of shock reversal, based on reduced vasopressor requirement
- High dose steroids for prolonged periods are harmful
- It remains unclear whether administering steroids as a bolus or as an infusion has comparable efficacy in the context of sepsis
- ADRENAL coming soon….Follow Critical Care Reviews Meeting 2018 #CCR18
- watch LIVE STREAM Friday, Jan 19th from 9am – 11am GMT

External Links
- [further reading] Bennett. The Effectiveness of Hydrocortisone in the Management of Severe Infections. A Double-Blind Study. JAMA. 1963;183(6):462-465
- [further reading] Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg 1976; 184:333–341
- [further reading] Sessler. Steroids for Septic Shock* Back From the Dead? (Con). CHEST 123 /5 / May 2003, Supplement 483S-489S
- [further reading] Sprung CL. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. NEJM 1984; 311:1137– 1143
- [further reading] The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. NEJM. 1987; 317:659–665
- [further reading] Bone RC. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. NEJM 1987; 317:653–658
- [further reading] Luce JM. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 1988; 138:62–68
- [further reading] Annane D 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871
- [further reading] Oppert. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005 Nov;33(11):2457-64
- [further reading] CORTICUS: Hydrocortisone Therapy for Patients with Septic Shock. Sprung et al. N Engl J Med 2008; 358:111-24
- [further reading] Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. COIITSS Study Investigators. JAMA. 2010 Jan 27;303(4):341-8
- [further reading] Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock. The VANISH Randomized Clinical Trial Gordon. JAMA. 2016;316(5):509-518
- [further reading] Hydrocortisone, Vitamin C and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Marik. CHEST 2016; published on line first Dec 2016
- [further reading] Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis. Keh. JAMA 2016. Published online October 3, 2016
- [further reading] Early initiation of low-dose hydrocortisone treatment for septic shock in adults: A randomized clinical trial. Qing-quan. American Journal of Emergency Medicine. 2017. Volume 35, Issue 12, Pages 1810–1814
- [further reading] Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. Annane. BMJ. 2004 Aug 28; 329(7464): 480
- [further reading] Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Sligl. Clin Infect Dis. 2009 Jul 1;49(1):93-101
- [further reading] Do low-dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systematic review and position statement prepared for the American Academy of Emergency Medicine. Sherwin. J Emerg Med. 2012 Jul;43(1):7-12
- [further reading] Wang. Low-dose hydrocortisone therapy attenuates septic shock in adult patients but does not reduce 28-day mortality: a meta-analysis of randomized controlled trials. Anesth Analg. 2014 Feb;118(2):346-57
- [further reading] Annane D, Bellissant E, Bollaert P, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No.: CD002243. DOI: 10.1002/14651858.CD002243.pub3
- [further reading] Gibbison. Corticosteroids in septic shock: a systematic
review and network meta-analysis. Critical Care (2017) 21:78 - [review] Farkas. PulmCrit (EMCrit). Steroids in septic shock: Four misconceptions and one truth
- [review] Long. emDOCs. The Controversies of Corticosteroids in Sepsis
Metadata
Summary author: Steve Mathieu
Summary date: 2nd January 2018
Peer-review editor: Charlotte Summers




It would appear that use of steroids in sepsis does not reduce 28 day mortality but instead provides a window of opportunity. I suggest that Dr Paul Marik’s use of Vitamin C and Thiamine makes the best current use of that window.
http://www.Isepsis.com
https://emcrit.org/category/isepsis/
The control in the Marik study was not placebo – it was a before-and-after study
Thanks for spotting the typo Chris – now corrected. BWs. Steve
Thanks for this summary. Thankfully, you have already covered ADRENAL and aprocchss. What about including the latest ycochrane review in your summary? https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002243.pub4/full
cheers for your great work! stay safe!