Early and Empirical High-Dose Cryoprecipitate for Hemorrhage After Traumatic Injury

CRYOSTAT-2
Davenport R, JAMA: Published online October 12th 2023 doi:10.1001/jama.2023.21019
Clinical Question
- In patients with trauma and critical bleeding who require activation of a major haemorrhage protocol, does the empiric administration of 3 pools of cryoprecipitate (6g fibrinogen) within 90 minutes of randomisation (and no more than 3 hours after injury) improve survival, compared to standard care?
Background
- The global burden of trauma is huge, with 4.4 million deaths worldwide per annum. It remains the leading cause of death in young people
- Deaths often occur early – > 50% of patients who die, do so in the first 4 hours
- The most common mechanism of death is haemorrhage, which is exacerbated by coagulopathy and fibrinolysis
- Fibrinogen acts to stabilise clot formation. Trauma causes activation of coagulation pathways and fibrinogen levels drop rapidly due to consumption, fibrinolysis and dilution
- There is an association between low fibrinogen levels and poor outcomes, and it is hypothesised that cryoprecipitate transfusion may ameliorate this state
- The ideal approach to transfusion in traumatic haemorrhage has been extensively studied and refined over the years
- The PAMPer trial demonstrated that for trauma patients at risk for hemorrhagic shock, the administration of thawed plasma during prehospital air medical transport was safe and resulted in lower 30-day mortality
- The CRYOSTAT pilot trial conducted at two UK trauma centres showed that administration of cryoprecipitate (10 units) in addition to the empiric massive haemorrhage pack (6u PRBC, 4u FFP +/- TXA) raised fibrinogen levels, and was feasible, with a mean time to administration of 60 minutes
Design
- Multi-centre, phase 3, interventional RCT
- Open-label, parallel-groups
- Allocation concealment achieved using opaque sealed envelopes
- The randomisation sequence was computer generated with variable block sizes and stratified by centre
- A waiver of consent was allowed in order to randomise patients
- Informed consent to continue data collection was obtained from the patient or legally authorised representative as soon as was practically possible
- The trial was designed to detect a 7% absolute mortality difference with 90% power and a 5% level of significance
- A sample size of 1600 patients was calculated adjusted after 4% drop out
- Analysis was by intention to treat
- The trial was overseen by an ethics committee and a data monitoring committee to review interim data and monitor patient safety
Setting
- 26 major trauma centres in the UK and USA.
- Patients were enrolled from August 2017 to November 2021
Population
- Inclusion:
- Trauma with evidence of active haemorrhage requiring activation of the local Major Haemorrhage Protocol (MHP)
- MHP activation at all centres included SBP < 90 mmHg at any point
- Started or received at least 1 unit of any blood component
- Trauma with evidence of active haemorrhage requiring activation of the local Major Haemorrhage Protocol (MHP)
- Exclusion:
- Transferred from another hospital
- Injuries deemed incompatible with life by trauma team leader
- >3 hours elapsed since the time of injury
- Participants:
- 1604 eligible patients, 799 were randomized to the cryoprecipitate group and 805 to the standard care group
- 1531 patients in intention-to-treat analysis due to missing data for 73 patients
- 1604 eligible patients, 799 were randomized to the cryoprecipitate group and 805 to the standard care group
- Baseline characteristics were well matched:
- Comparing cryoprecipitate group vs standard care:
- Median Injury Severity Score: 29 vs 29
- Men: 79 vs 80%
- Median Age: 38 vs 40
- 10% > 70 years old
- Median time from injury to ED arrival: 75 vs 77 minutes
- Blunt injury: 63 vs 65%
- Penetrating injury: 37 vs 35%
- Head injury (AIS >4): 24% vs 29%
- Prehospital Tranexamic Acid: 79 vs 80%
- Prehospital Blood Products: 43% across both groups
- Median time to randomisation: 15 minutes across both groups
Intervention
- 3 pools of cryoprecipitate (6g fibrinogen) given as early as possible (ideally within 90 minutes) after hospital admission AND MHP as per control group
- 85% of patients got cryoprecipitate in the intervention group
- The main reasons for not receiving it were no evidence of active bleeding, haemostasis was achieved or the patient died
- Median time to cryoprecipitate was 68 minutes (68% received the first dose within 90 minutes)
Control
- As per local MHP – typically patients were transfused RBC:FFP:Platelets in a ratio of 1:1:1 with 2 pools cryoprecipitate with second and subsequent pack (4g fibrinogen)
- 32% of patients received cryoprecipitate in the control group
- Median time to cryoprecipitate was 120 minutes (9% received the first dose within 90 minutes)
Management common to both groups
- All other therapies were as per local major trauma protocols
Outcome
- Comparing Cryoprecipitate vs Standard Care
- Primary outcome:
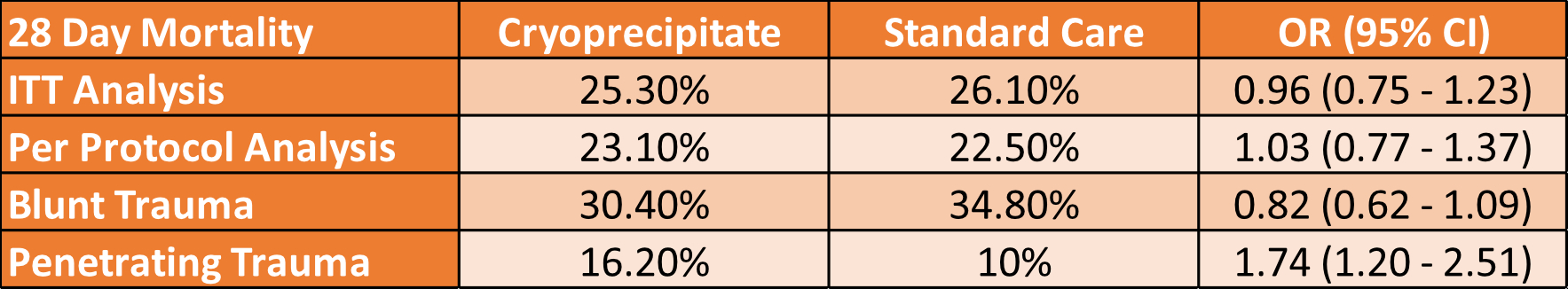
- No difference in all-cause mortality at 28 days
- 25.3% (192 of 760) vs 26.1% (201 of 771)
- OR 0.96 [95% CI 0.75-1.23] p=0.74
- The per-protocol analysis also showed no difference
- Secondary outcomes:
- No difference between groups in:
- 6-hour mortality: 7.1% vs 8.6% (OR 0.82 [0.58-1.17] p=0.26)
- 24-hour mortality: 11.2% vs 12.2% (OR 0.91 [0.63-1.31] p=0.61)
- 6- or 12-month mortality
- Transfusion Requirements, Ventilator Days, ICU LOS, Hospital LOS, GOS score at day 28
- Median time to death was 191 minutes in the cryoprecipitate group and 86 minutes in the control group
- An analysis of the impact of time to cryoprecipitate administration on 28-day mortality (eFigure2):
- < 45 minutes: OR 1.45 [0.91 – 2.31]
- 46-60 minutes: OR 1.16 [95% CI, 0.78-1.73]
- 61-90 minutes: OR 0.57 [95% CI, 0.38-0.87]
- > 90 minutes: OR 1.00 [95% CI, 0.62-1.60]
- Pre-defined subgroups:
- No difference in results based on sex, age, head injury
- The penetrating injury group fared worse with cryoprecipitate
- 28-day mortality 10% vs 16.2% OR 1.74 [1.2-2.51]
- The blunt injury group had 28-day mortality of 30.4% (Cryo) vs 34.8% (Standard Care) OR 0.82 [0.62-1.09]
- Safety:
- No significant difference in
- Thrombotic events (PE, DVT)
- Myocardial infarction, Stroke
- No significant difference in
Authors’ Conclusions
- Early empiric high dose cryoprecipitate in patients with haemorrhage following trauma did not reduce 28-day mortality
Strengths
- Strong internal validity:
- Allocation concealment
- Intention-to-treat analysis
- Well balanced baseline characteristics
- Minimal loss for primary analysis
- 4.6% (39 patients in cryoprecipitate group and 34 patients in control group)
- An important clinical question with outcomes that are clinically meaningful
- Patients had high injury severity scores thus increasing the chance of finding a treatment effect
- The results are likely applicable to other similar advanced trauma care systems
- However, this was primarily a trial in UK hospitals, with only 49 US patients randomised so the external applicability outside the UK would need to be assumed cautiously
Weaknesses
- Unblinded – a potential source of bias
- There was some crossover between groups
- 15% of cryoprecipitate group did not receive any
- 9% of the control group got early cryoprecipitate (within 90 minutes)
- This was not deemed to impact the results in the per-protocol analysis
- The intervention was empiric and not tailored to the individual’s needs such that some patients may not have had hypofibrinogenemia
- Giving cryoprecipitate in these patients with normal fibrinogen may be pro-inflammatory and harmful
- There is a signal to benefit in the blunt trauma subgroup and harm in the penetrating subgroup. However, there is inadequate statistical power to interpret these subgroup findings
The Bottom Line
- The results from this trial do not support empiric cryoprecipitate therapy in trauma patients with major haemorrhage
- The hypothesis that there is a Goldilocks zone (i.e. 61-90 minutes) in which administration of cryoprecipitate may be beneficial is thought-provoking and warrants further exploration
- I will use ROTEM to guide product administration in my bleeding trauma patients
External Links
- Article CRYOSTAT-2
- Further reading PATCH trial
- Further reading Centre for Trauma Sciences
- Further reading St Emlyn’s review
- Critical Care Reviews Presentation
Metadata
Summary author: Celia Bradford @celiabradford
Summary date: October 14 2023
Peer-review editor: George Walker
Picture by: Clker-Free-Vector-Images / Pixabay