ATACH-2 Trial
Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage
Qureshi et al. NEJM 2016 DOI: 10.1056/NEJMoa1603460
Clinical Question
- In patients with acute intracerebral haemorrhage and who are hypertensive, does rapid lowering of systolic blood pressure compared to standard therapy improve patient outcomes ?
Design
- Randomised, multi-centre, open-label
- Central randomisation using trial website by minimisation algorithm combined with biased-coin method
- Follow up period of 3 months
- Power calculation based on assumption that death or disability would be 10% lower in intervention group. Expected control event rate of 60%.
- alpha error 0.05, power 90%
- Sample size of 1280 required taking into account non-adherence
- Intention-to-treat analysis
Setting
- 110 sites in United States, Japan, China, Taiwan, South Korea and Germany
- May 2011 to September 2015
Population
- Inclusion:
- 18 years and above
- GCS > 4
- Intraparenchymal haematoma of less than 60cm3 on initial CT scan
- Within 3 hours (extended to 4.5 hours) of symptom onset
-
At least one reading of systolic blood pressure of 180 mmHg or more between symptom onset and the initiation of intravenous antihypertensive treatment
- Exclusion:
- Time of symptom onset not reliably established
- Previously known AVM, neoplasm or aneurysm
- Intracerebral hematoma considered to be related to trauma.
- lCH located in infratentorial regions such as pons or cerebellum
- IVH associated with intraparenchymal hemorrhage and blood completely fills one lateral ventricle or more than half of both ventricles
- Subject is considered a candidate for immediate surgical intervention by the neurosurgery service
- Pregnancy or parturition within previous 30 days or active lactation
- Any history of bleeding diathesis or coagulopathy
- Use of warfarin within the last 5 days
- A platelet count less than 50,000/mm3
- Known sensitivity to nicardipine
- Pre-morbid mRS of 4 or greater
- Subject’s living will precludes aggressive ICU management
- 8532 patients screened, 1000 randomised
Intervention
- * Treatment could be initiated before randomisation to lower the systolic blood pressure to less than 180 mmHg but were excluded if systolic BP reduced below 140 mmHg before randomisation
-
Target systolic BP 110 to 139 mmHg throughout the 24 hours after randomisation
Control
- Target systolic BP 140 to 179 mmHg throughout the 24 hours after randomisation
Management common to both groups
- 1st line = IV nicardipine infusion
- initiated at 5mg/hr, increased by 2.5mg/hour every 15 minutes as needed, up to a maximum dose of 15mg/hr
- 2nd line (if target not achieved after 30 mins) = IV labetalol (IV diltiazem or urapidil if labetolol not available)
Outcome
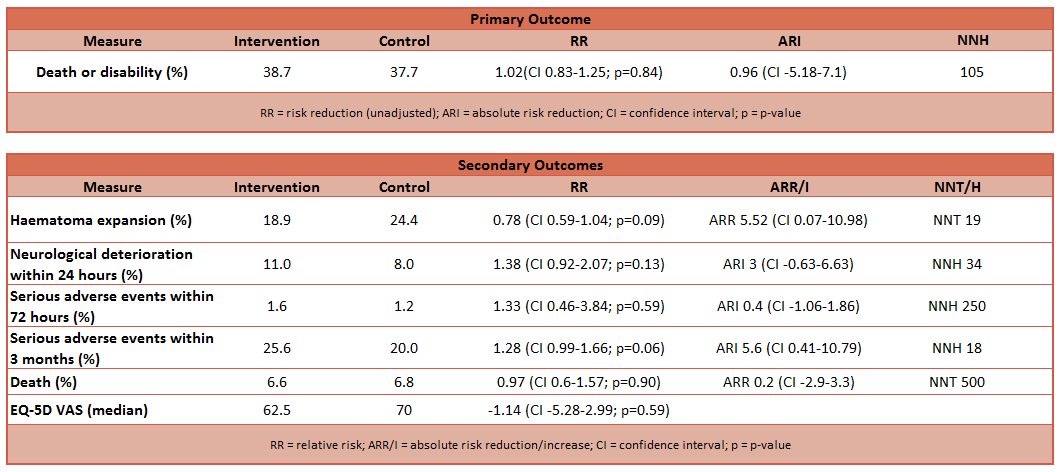
- Primary outcome:
- Proportion of patients who had modified Rankin scores 4 to 6 at 3 months (significant disability or death)
- No difference between groups (38.7% in intervention, 37.7% in control)
- Unadjusted analysis: relative risk 1.02 (CI 0.83 – 1.25, p=0.84)
- Adjusted analysis: relative risk 1.04 (CI 0.85 – 1.27, p=0.72)
- No difference between groups (38.7% in intervention, 37.7% in control)
- Proportion of patients who had modified Rankin scores 4 to 6 at 3 months (significant disability or death)
- Secondary outcome:
- Unadjusted analysis showed no difference between groups in:
- EQ-5D scores at 3 months
- VAS at 3 months
- Proportion of patients with expansion of 33% or more in volume of haematoma on 24 hour scan compared to baseline scan
- Safety outcomes (fall in GCS by 2 or increase of NIH Stroke Score by 4)
- Incidence of serious adverse events within 72 hours
- Higher incidence of serious adverse events within 3 months in intervention group when analysis adjusted
- 25.6% in intervention, 20% in control; p=0.05
- Unadjusted analysis showed no difference between groups in:
- Primary treatment failure = not achieving target within 2 hours after randomisation
- 61 (12.2%) in intervention arm, 4 (0.8%) in control arm; p<0.001
- Secondary treatment failure = hourly minimum systolic BP above target range for 2 consecutive hours during the period of 2 to 24 hours after randomisation
- 78 (15.6%) in intervention arm, 7 (1.4%) in control arm; p<0.001
 Authors’ Conclusions
Authors’ Conclusions
- The results of the trial do not support the reduction of systolic blood pressure to a target of 110 to 139 mmHg in patients with intracerebral haemorrhage
Strengths
- Multi-centre trial
- Clinically meaningful and relevant outcome measures
Weaknesses
- Recruitment criteria changed during the trial
- Reasons for failure to randomise screened population not included
- Under-powered – power calculations based on event rate of 60%, actual observed event rate in study of 38%, increasing likelihood ot type 2 error
- More than 50% of patients recruited from Asia sites and may not be reflective of European population
- Intracerebral haemorrhage grouped as a single entity
- Significant difference between groups with regards to treatment failure (primary and secondary)
The Bottom Line
- The results of this trial and that of the INTERACT2 trial do not support an early, intensive control of systolic blood pressure in patients with acute intracranial haemorrhage
External Links
- [article] Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage
- [further reading] Supplementary material
- [further reading] Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage (INTERACT2)
Metadata
Summary author: Adrian Wong
Summary date: 15 July 2016
Peer-review editor: Duncan Chambler




The INTERACT-2 trial that had a small benefit in functional outcome (secondary outcome), and also had a later published subgroup analysis that had less hematoma growth with intensive BP management (<140 vs <180). How do we reconcile the divergent bottom lines of these two studies?
Pingback: SGEM#172: Don’t Bring My Blood Pressure Down (Intensively) – The ATACH2 Trial | The Skeptics Guide to Emergency Medicine
Pingback: SGEM #172: Spanish – No se traiga mi presión arterial abajo (intensivamente): El Estudio ATACH2 | The SGEM Global