PLUS Study

Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults
Finfer S, NEJM, 2022; DOI: 10.1056/NEJMoa2114464
Clinical Question
- In critically ill adults does the use of a balanced multielectrolyte solution (BMES, Plasmalyte 148), as opposed to 0.9% Sodium Chloride, for fluid resuscitation and therapy result in reduced 90 day mortality?
Background
- Fluid administration is a key component of critical care therapy
- If one fluid is superior to another, even by the smallest margin, the global impact on critical care outcomes will be significant
- Saline has long been the most commonly administered fluid in the ICU, but there have been concerns that a hyperchloraemic acidosis may induce renal impairment, although recent trials have had conflicting outcomes
- The most recently published BaSICS trial showed no difference in 90-day mortality between Plasmalyte and 0.9% Sodium Chloride in 11000 adult intensive care patients
- The SMART trial involving 15000 adult ICU patients in 2018 showed a reduction in a composite outcome of death, new renal replacement therapy and persistent renal dysfunction at 30 days, with balanced crystalloid use
- The SPLIT trial in 2015 compared Plasmalyte to 0.9% Sodium Chloride in 2278 critically ill patients. There was no difference in the primary outcome of proportion of patients with an acute kidney injury.
- Although not statistically significant, point estimates favoured Plasmalyte with regards to the secondary outcomes of RRT requirement and death in hospital
- The repeated signals favouring balanced crystalloids have prompted investigators to conduct trials with adequate power.
Design
- Investigator initiated, double-blind, parallel-group, randomised, controlled trial
- Block randomisation with variable block sizes
- Randomisation conducted via secure website
- Study treatments supplied in identical 1000ml bags that were macroscopically indistinguishable
- Data collected daily for first 7 days included haemodynamic variables, urine output, organ support, laboratory data and volume of fluids administered, data collected daily through to day 90 included ventilations and RRT status, creatinine levels and volume of trial fluid administered
- Patients followed up for 6 months post randomisation
- Analysed on intention to treat basis
- Analysis was unadjusted, adjusted, adjusted by multiple imputation for missing data and a post-hoc inverse-probability-weighting analysis of the primary outcome to account for patients in the BMES group who received open-label saline after randomization was conducted
- Predefined subgroups (AKI, APACHE II score and post-surgical admission) and sensitivity analyses in addition to planned cost-effectiveness analysis
- Power calculation
- 8800 required with 90% power to detect a 2.9% absolute reduction in 90 day mortality at an alpha of 0.05 (assumed 23% mortality in control group) with a ~2% loss to follow up
- Based on SPLIT (risk reduction) and NICE SUGAR (mortality rate with a 2% reduction for improvement in mortality since 2008)
- COVID-19 disrupted recruitment and many sites had non-COVID-19 clinical research suspended and further funding to continue for longer not available
- Power calculations revised:
- ~5000 patients provides 90% power to detect a 3.8% absolute difference in 90 day mortality
- Power calculations revised:
- 8800 required with 90% power to detect a 2.9% absolute reduction in 90 day mortality at an alpha of 0.05 (assumed 23% mortality in control group) with a ~2% loss to follow up
- Appropriate ethical approval and consent processes
- Pre-published method and statistical analysis plan, which was amended in 2019 when the sample size was revised
Setting
- 53 ICUs in Australia (41) and New Zealand (12)
- September 2017 – December 2020
Population
- Inclusion:
- Requirement for fluid resuscitation
- This was defined as a bolus of fluid administered over one hour or less to increase or maintain intravascular volume in addition to maintenance fluids
- Requirement supported by at least one of seven pre-specified clinical signs (HR > 90bpm, SBP < 100 mmHg, MAP < 75 mmHg, CVP < 10, CRT > 1 sec, UO < 0.5 ml/kg/hr for at least one hour)
- Expected to be in ICU on three consecutive days
- CVC or arterial line in situ (or imminent placement planned)
- Clinical equipoise between PL148 and 0.9% Sodium Chloride
- Requirement for fluid resuscitation
- Exclusion:
- < 18 yo
- Prior fluid resuscitation > 500ml in current ICU admission
- Contraindication to study fluid
- Those admitted with specific fluid requirements (e.g. burns)
- TBI, or at risk of cerebral oedema
- Death deemed imminent, or an underlying disease process with life expectancy < 90 days
- Known or suspected pregnancy
- Prior enrolment in PLUS
- 5037 randomised
- 2515 BMES and 2522 to saline
- 16828 screened
- 11791 excluded
- Majority due to exclusion criteria
- 705 due to “other”
- 247 due to “ICU clinician declines enrolment”
- 11791 excluded
- Comparing baseline characteristics of BMES vs. Saline group
- Age: 61.7 vs 62.1
- Female: 37.3% vs 40.1%
- ICU admission source:
- ED: 34.0% vs 31.8%
- Emergency Surgery: 26.8% vs 26.8%
- Elective Surgery: 17.8% vs 19.0%
- Median APACHE II: 19 vs 19
- Sepsis (Sepsis-3): 43.8% vs 42.6%
- Trauma Admission: 8.2% vs 8.7%
- New RRT: 1.9% vs 2.2%
- Invasive Mechanical ventilation: 75.9% vs 76.8%
- Creatinine (micromol/L): 127.4 vs 125.9
- Chloride (mmol/L): 105.4 vs 105.6
- pH: 7.3 vs 7.3
- Lactate: 2.7 vs 2.7
- Received > 500mls of alternate fluid in 24 hours prior to randomisation: 55.5% vs 23.2%
- Median 625mls of saline received by BMES group
- Clinical Parameters prior to randomisation (HR, MAP) similar
- Median SOFA score (respiratory and cardiovascular domain) similar
- Median time from ICU admission to randomisation (hours): 2 vs 2
Intervention
- Plasmalyte 148
Control
- 0.9% Sodium Chloride
Management common to both groups
- Patient received assigned study fluid for all episodes of resuscitation and compatible IV crystalloid therapy in ICU until 90 days following randomisation
- If study fluid was non-compatible with any drugs, then preferably 5% glucose used wherever possible to minimise exposure to alternate fluid
- If specific crystalloid solutions clinically indicated these were allowed
- If readmitted within 90 days then use of study fluid continued
- All other treatment at discretion of treating clinicians
Fluid administered post randomisation
- Trial fluid administered in 96.0% (BMES) and 96.2% (Saline) of patients
- Median duration of treatment with assigned fluid:
- 6 vs 6 days
- Median volume of fluid received:
- 3.9L (BMES) vs 3.7L (Saline)
- Post randomisation, >500ml alternate fluid administration:
- 63.0% (BMES) vs 3.5% (Saline)
- Mean difference in serum chloride -1.99 mmol/L (95% CI -2.21 – -1.76)
Outcome
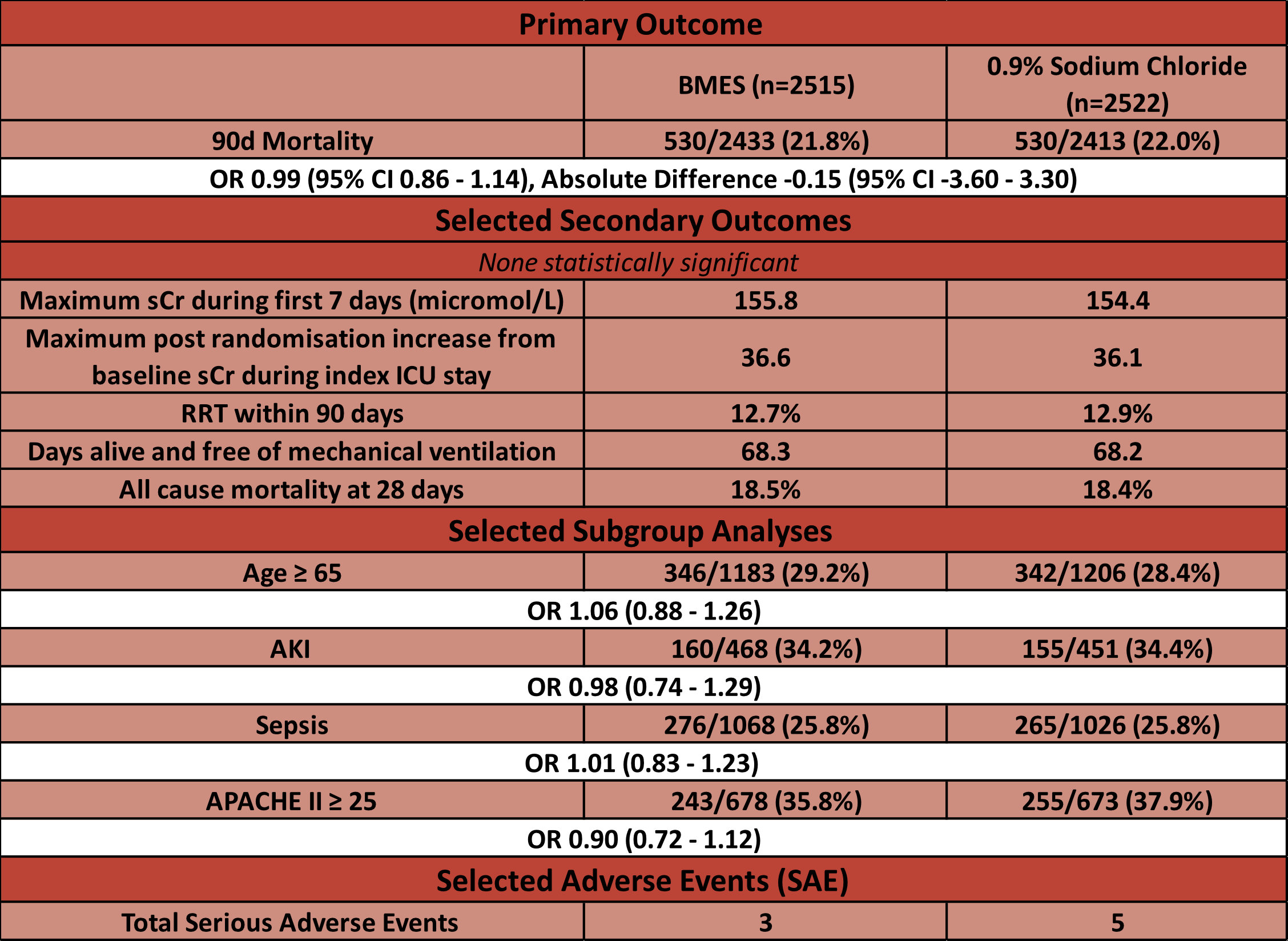
- Primary outcome:
- Unadjusted 90-day mortality (BMES vs Saline)
- 21.8% v 22.0% (530 deaths in each group)
- ARR -0.15% (95% CI -3.6 – 3.3)
- Secondary analyses showed no change in primary outcome when those receiving > 500ml of alternate fluid either prior to randomisation or in the ICU post randomisation were excluded
- Post-hoc analysis which aimed to account for those in BMES group who received open label saline also showed no difference in primary outcome
- Secondary outcomes:
- Comparing PL-148 vs. Sodium Chloride
- No significant difference in:
- New RRT
- Receipt of vasoactive drugs
- Days alive and free of mechanical ventilation
- Maximum creatinine level or increase in creatinine
- No significant difference in:
- Subgroup Analyses:
- No statistically significant differences noted in any of pre-specified subgroups
Authors’ Conclusions
- The use of PL-148 compared to 0.9% Sodium Chloride does not reduce 90-day all cause mortality or risk of AKI
Strengths
- Large, randomised trial across 53 ICUs in 2 countries
- Protocols for randomisation and masking of treatments minimise selection and detection bias
- Pre-published statistical analysis plan and intention to treat analysis
- 96% follow up to day 90
- Patients appear unwell with ~75% needing invasive mechanical ventilation, and low proportion of elective surgical patients
- For comparison elective surgical rates in other trials: BaSICS 48%, SPLIT 57%, Not reported in SMART
- Larger volumes of trial fluid administered compared to other trials (~4L in 6 days)
- For comparison: BaSICS 2.9L, SPLIT 2L, SMART 1L
- Prior compatibility testing for Plasmalyte 148 and 87 drugs important to minimise contamination bias
- Results consistent with recently published BaSICS trial
- Given the extra cost associated with BMES the decision to collect economic data is important
Weaknesses
- Reduction in recruitment size
- This was unavoidable given pressures external to the trial. Given the futility boundary was crossed at a similar time point to trial cessation it’s highly unlikely that the results would have changed if the trial had continued
- 63% in BMES (as opposed to 3.5% in saline group) received > 500ml of alternate fluid may result in a contamination
- Figure 1 shows roughly 200mls/day of “open-label” saline administered to each group
- There were a large number of protocol deviations (Table S5)
- Resuscitation / Fluid Bolus:
- In BMES group non study fluid administered in 10.8% patients (6.8% albumin, 1.7% saline)
- In Saline group non study fluid administered in 11.5% (7.3% albumin, 1.9% BMES)
- Maintenance Fluid:
- In BMES group non study fluid administered in 6.1% patients (2.3% saline)
- In Saline group non study fluid administered in 5.7% (1.3% BMES)
- Resuscitation / Fluid Bolus:
- Fluids not controlled or recorded outside of ICU
The Bottom Line
- This high quality, large, randomised trial provides no evidence that 0.9% Sodium Chloride causes harm
- This trial was published alongside a large meta-analysis of ~35000 patients (including this trial) in which the estimated effect of BMES vs 0.9% Sodium Chloride ranged from a 9% relative reduction to 1% relative increase of death by day 90. In sepsis, the use of BMES was even more pronounced with a range from 14% relative reduction to a 1% increase
- Although these confidence intervals are not statistically significant, my personal preference will be to continue to use balanced crystalloids in non neuro-ICU patients
External Links
- [article] Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults
- [meta-analysis] Balanced Crystalloids versus Saline in Critically Ill Adults — A Systematic Review with Meta-Analysis
- [further reading] Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA)
- [Livestream] Critical Care Reviews Livestream
Metadata
Summary author: @hgmwalker89
Summary date: 19th January 2022
Peer-review editor: @celiabradford
Picture by: iStock




Thank you for the review of the Plus Study.
I have one question:
One exclusion criteria was: Prior fluid resuscitation > 500ml in current ICU admission
In the supplemental you can find that about 4300 patients were excluded based on that.
But: 55.5% vs 23.2% received > 500mls of alternate fluid in 24 hours prior to randomisation
Median 625mls of saline received by BMES group.
Can you explain that?
Thanks
Christoph
Hi Christoph. My understanding is that they didn’t control for fluid before ICU admission (hence the alternate fluid) but if on arrival to ICU they were deemed to require further IV fluid (and required > 500mls) then this was an exclusion criteria. I hope this helps.
Thanks for your response,
Now I understand it much better.
But ignoring the volume of fluid administrated prior ICU admission is a relevant bias for outcome.