VAM-IHCA
Effect of Vasopressin and Methylprednisolone vs Placebo on Return
of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest:
A Randomized Clinical Trial
Anderson et al. JAMA 2021. doi:10.1001/jama.2021.16628
Clinical Question
- In adult patients suffering an in-hospital cardiac arrest (IHCA) does the use of vasopressin and methylprednisolone compared to a placebo result in increased rates of return of spontaneous circulation (ROSC)?
Background
- IHCA in the UK has an occurrence of 1:1000 admissions (~13000 per annum) and is poorly studied compared to out-of-hospital cardiac arrest (OOHCA) with few large scale trials
- IHCA has been described as a different entity to OHCA
- Different aetiologies and often shorter time to recognition and effective CPR (and defibrillation if appropriate)
- It has been reported that further strategies are needed to improve outcomes
- In 2013, the VSE trial showed improved survival to hospital discharge with favourable neurological outcome with the addition of vasopressin and steroids alongside adrenaline
- It has been shown that endogenous vasopressin levels are lower in non-survivors of cardiac arrest
- Likewise, cortisol levels have been demonstrated to be higher in those successfully resuscitated
- It is thought this might relate to the cardiovascular effects of glucocorticoids such as increased sensitivity, increased production of enzymes involved in catecholamine synthesis and inhibition of breakdown
- A 2019 Cochrane Review concluded that vasopressin compared to standard dose adrenaline in OOHCA improved survival to admission but not ROSC, whereas the combination of vasopressin and adrenaline had no effect on these outcomes
Design
- Investigator-initiated, multi-center, randomized, placebo-controlled, parallel group, double-blind, superiority trial
- Randomised in a 1:1 ratio
- Random blocks of 2,4 or 6 used and blocks stratified by site
- Trial drugs brought to patient in blinded study kit by a dedicated member of the clinical cardiac arrest team
- Pre-specified secondary and safety outcomes alongside 5 subgroups
- Clinical data (SOFA score) collected at 24,48 and 72 hours post IHCA
- Neurological outcomes assessed at 30,90, 180 days and 1 year post IHCA
- Sample size calculation required 492 patients
- alpha of 0.05 and power 80%
- Based on unpublished data showing IHCA ROSC rate of 45% in placebo group and 13% absolute difference in ROSC rates between groups
- Consent sought from patients or surrogates if survived
- Temporary consent gained from a physician independent of trial
- Independent data monitoring committee oversaw trial
Setting
- 10 hospitals in Denmark
- October 2018 – January 2021
Population
- Inclusion:
- > 18 yo
- IHCA
- Received at least 1 dose adrenaline
- Exclusion:
- Valid DNA-CPR
- Prior enrolment
- Invasive circulatory support
- Known or suspected pregnancy
- 2362 screened –> 1850 excluded
- 512 randomised
- 11 excluded as did not meet inclusion criteria
- 501 analysed
- 237 intervention
- 264 placebo
- Baseline characteristics well balanced (comparing vasopressin/methylprednisolone vs. placebo)
- Age: 71 v 70
- Male: 62% v 66%
- Rates of baseline co-morbidities (hypertension, coronary artery disease, diabetes etc) similar
- Glucocorticoids prior to IHCA: 14% v 11%
- Majority located on hospital ward: 69% v 64%
- ICU: 10% v 7%
- Monitored: 37% v 46%
- Witnessed: 71% v 77%
- Shockable: 9 v 11%
- Majority PEA: 57% v 52%
- Time to study drug administration: 8 v 9 mins
- Doses of drug/placebo:
- 1: 28% v 28%
- 2: 32% v 27%
- 3: 15% v 18%
- 4: 24% v 27%
- Adrenaline doses: 3 v 3
- Intubation during cardiac arrest: 74% v 68%
- If survived > 24 hours:
- TTM: 26% v 27%
- Coronary Angiography: 24% v 25%
- VA ECMO: 14% v 30%
- Glucocorticoid use: 24% vs 46%
Intervention
- 40mg Methylprednisolone and 20IU vasopressin
- Given ASAP after first adrenaline dose
- Further doses of vasopressin (20IU, to a maximum 4 doses) could be given after each further adrenaline doses
Control
- 0.9% Sodium chloride
- Ampoules identical
Management common to both groups
- All other management at discretion of treating teams
Outcome
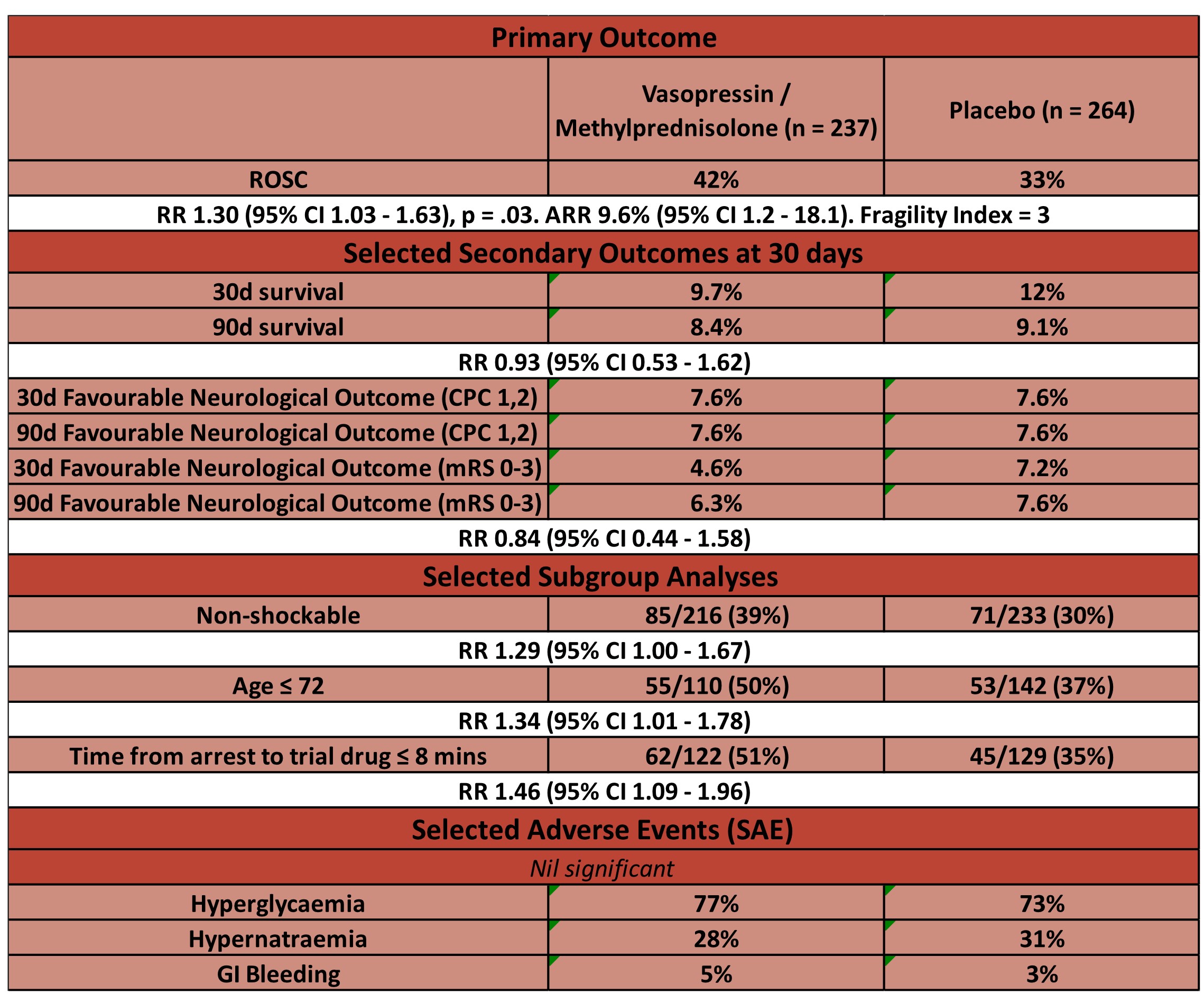
- Comparing vasopressin/methylprednisolone vs. placebo
- Primary outcome:
- ROSC
- 42% vs 33% – significantly higher in vasopressin/methylprednisolone group
- RR 1.30 (95% CI 1.03 – 1.63), p = .03
- Fragility Index = 3
- ROSC
- Secondary outcomes:
- No significant difference at 30 or 90 days in
- Survival
- Favourable neurological outcome
- No significant difference at 30 or 90 days in
- Safety Outcomes
- Hyperglycaemia: 77% v 73%
- Hypernatraemia: 28% v 31%
- Pneumonia: 21% v 17%
- GI bleeding: 5% v 3%
- In those that survived to 30d or had favourable neurological outcome at 30 days no benefit shown in any subgroup
Authors’ Conclusions
- The administration of vasopressin and methylprednisolone, compared
to a placebo, significantly increased the likelihood of
return of spontaneous circulation - Uncertainty remains as to whether this treatment results in long term survival benefit or harm
Strengths
- Important question to answer given previous benefits shown in trials in an area with few trials with large numbers recruited
- A large trial in a logistically challenging area
- IHCA are often fraught environments and enrolling, randomising and administering study drug within 9 minutes is notable
- Good internal validity
- Multicentre
- Double blinded, placebo controlled
- Good randomisation strategy
- Balanced baseline characteristics
- Good adherence to protocol
- 18 deviations in vasopressin / methylprednisolone group vs 19 in placebo
- Pre-published protocol
- Sensible primary outcome
- The use of adrenaline in cardiac arrest algorithms is only based on increased rates of ROSC and 3 month survival
- Its use does not alter long term survival or survival with a favourable neurological outcome
- The authors note that to adequately power a study to detect a 2% difference in neurological outcome would require > 7000 patients
- Rates of ROSC may not seem patient focused however:
- Families might value this time as a chance to say goodbye
- There were increased rates of organ donation in the PARAMEDIC2 trial
- The use of adrenaline in cardiac arrest algorithms is only based on increased rates of ROSC and 3 month survival
- It seems reasonable to not place too much weight on the neurological outcome given:
- It was not powered to detect this
- There are some discrepancies between the various scoring tools of neurological outcome:
- The rates of CPC 1 and 2 at 90 days were identical, this is contrast to the point estimate favouring harm for the mRS score at both 30 and 90 days (however neither were significant)
- The EQ-5D-5L (a health related QoL metric) point estimates were higher in the vasopressin / methylprednisolone group at both 30 and 90 days (again neither significant)
Weaknesses
- High numbers excluded “for other reasons”
- Mostly clearly documented with valid reasons however 170 (7% of those screened) excluded due to “physician preference” and in 193 (8% of those screened) “team forgot”
- This may introduce a selection bias
- Mostly clearly documented with valid reasons however 170 (7% of those screened) excluded due to “physician preference” and in 193 (8% of those screened) “team forgot”
- Only performed in one country (Denmark)
- Rates of ROSC lower than study was powered for
- Achieved 33% in control, powered for a 45% rate of ROSC
- This may introduce a type 1 error
- In prior study that showed improved neurological function, steroids were continued for 7 days following ROSC
- In those surviving > 24 hours there were higher rates of glucocorticoid use in the placebo group
- Higher rates of extracorporeal support in placebo group
- Low rates of TTM overall (27 v 26%)
- Despite the TTM2 trial results there is no data provided as to the number who were febrile. Additionally, the TTM trials studied OOHCA and no trial is yet to compare TTM to no TTM
- Overall 30d survival low
- The UK 2019/2020 National Cardiac Arrest Audit showed 24% survival to hospital discharge. This is similar to a systematic review of IHCA outcomes in ANZ
- Theoretically those patients with normal conduction (i.e. PEA) may benefit more from high doses of vasopressors as opposed to shockable rhythms
- This may be evidenced by the subgroup analysis looking at shockable vs non-shockable
- This study had high rates of PEA (57% v 52%) so may not be as applicable to IHCA cohorts that have higher rates of shockable or asystolic rhythms
The Bottom Line
- At present there is insufficient evidence to include the use of vasopressin and methylprednisolone as standard care in the management of IHCA
- However, given the evidence base for the use of adrenaline in cardiac arrest algorithms it will be interesting to see the recommendations of expert committees if further trials demonstrate a survival benefit
External Links
- [article] Trial
- [editorial] Vasopressin and Steroids as Adjunctive Treatment for IHCA
- [further reading] European Resuscitation Council 2021 Guidelines
- [further reading] In-Hospital Cardiac Arrest: A Review
- [podcast] Critical Care Reviews: VAM-IHCA
Metadata
Summary author: George Walker @hgmwalker89
Summary date: 9th October 2021
Peer-review editor:David Slessor
Picture by: Pexels [Allec Gomes]





Rates of ROSC lower than study was powered for. Why does this introduce a type 1 error? Doesn’t it introduce Beta (type 2) error? I refer to page 36 of the trial protocol (figure 1)