IMPACT

A factorial trial of six interventions for the prevention of postoperative nausea and vomiting
Apfel. NEJM 2004;350:2441-2451. doi:10.1056/NEJMoa032196
Clinical Question
- In adult patients at risk of nausea and vomiting during surgery, do multiple antiemetic interventions compared to single antiemetic interventions or no intervention reduce nausea and vomiting?
Background
- Whilst not a glamorous topic, nausea and vomiting after surgery is known to be an unpleasant experience for patients and is often rated as worse than post-operative pain
- Given the number of patients undergoing surgery globally and the high incidence of post-operative nausea and vomiting (PONV), good treatment and prevention of symptoms will have huge impact on patient experience, outcomes and hospital resource consumption
- This complex multi-factorial trial is a classic paper that has formed a framework for today’s clinical care and the training of tomorrow’s anaesthetists
Design
- Multi-centre, randomised controlled trial
- 6 interventions tested in a 2x2x2x2x2x2 factorial design
- This allowed comparison of each individual intervention and the effect from combining any combination of interventions
- Total 64 possible allocation groups!
- Randomisation sequence was computer generated in stratified blocks
- Allocation was concealed using opaque envelops opened after recruitment and before induction of anaesthesia
- Anaesthetists providing care were not blinded to group allocation, but patients, post-operative care providers and outcome assessors were blinded to allocation
- Sample size calculations are not described in detail in the manuscript, but it is stated that 5000 patients would provide adequate power to compare any single intervention or any combination of 2, 3 or 4 interventions against the control groups
- Modified intention-to-treat analyses, excluding patients with incomplete outcome data (4.4%)
Setting
- 28 centres from Turkey, Germany, Finland, Austria, Slovakia, Spain and UK
- February 2000 to July 2002
Population
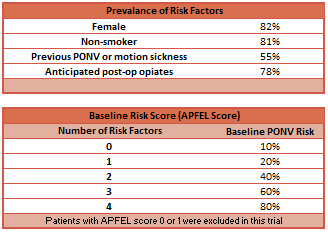
- Inclusion: Adults undergoing elective surgery with expected duration > 60 minutes + risk of PONV > 40% (APFEL Score ≥ 2)
- Exclusion: study drugs were contraindicated; taken emetogenic or antiemetic drugs within 24 hours of surgery; anticipated to need post-operative mechanical ventilation; pregnant or lactating
- 5199 randomised; 5161 analysed for four factors; 4086 analysed for all six factors
- 414 not randomised with regards to oxygen + air / oxygen + nitrous oxide allocation
- 191 not randomised with regards to remifentanil / fentanyl allocation
- 181 not randomised with regards to both carrier gas and opiate allocation
- 227 patients (4.4%) had incomplete outcome data and were not included in analysis
- Baseline variables were similar between groups
- See table for prevalence of risk factors for PONV
- Surgical procedures were represented by
- Gynaecology 45%
- General surgery 31%
- orthopaedic 15%
- Head and neck 9%
Intervention
- Multi-factorial antiemetic interventions
- Ondansetron 4mg IV within last 20 minutes of surgery
- Dexamethasone 4mg IV within 20 minutes of induction
- Droperidol 1.25mg IV within 20 minutes of induction
- Propofol maintenance starting at “80 mcg/kg/hour”
- Remifentanil intra-operatively 0.25 mcg/kg/min and increased as required, followed by 50 mcg/kg morphine at the end of surgery
- Oxygen and air (with two possible sub-allocation in some trial centres)
- 80% oxygen
- 30% oxygen
Control
- Non-administration of the antiemetic interventions
- No dexamethasone
- No ondansetron
- No droperidol
- Volatile maintenance at “standardized concentration”
- Fentanyl intra-operatively 100–200 mcg at induction and further bolus of 50–100 as required
- Oxygen and nitrous oxide
- 30% oxygen only
Factorial design
- For each antiemetic intervention, patients were randomly allocated to receive the intervention or the matched control
- This created six random allocations for each patient
- For example, regarding the dexamethasone intervention, a patient would have been randomised to receive dexamethasone or not receive dexamethasone
- Regarding propofol maintenance, patients were randomised in a 2:1 ratio to maintain the power of the statistical testing of this intervention
- Regarding carrier gas, patients were randomised in three centres in a 1:1:1 ratio to allow analysis of high and low concentrations of oxygen when mixed with air as a substudy
Management common to both groups
- All patients received a standardized anaesthetic with the exception of the interventions listed above
- Pre-medication with benzodiazepine
- Opiate (remifentanil or fentanyl) commenced 3 minutes before propofol 2–3 mg/kg induction
- Tracheal intubation
- Rocuronium
- Normocapnic mechanical ventilation
- Maintenance (propofol or volatile) could be titrated at anaesthetists discretion
- Opiates increased if heart rate or blood pressure deviated by +20% from baseline
- Post-operative care was guided
- Supplemental oxygen
- NSAID analgesics
- Opiates at the discretion of the anaesthetist
- Rescue antiemetics given in standardized order:
- Ondansetron 4mg
- Dexamethasone 4mg
- Droperidol 1.25mg
Outcome
- Primary outcome: the incidence of any nausea, vomiting or both was assessed during the first 24 hours after surgery
- Ondansetron, Dexamethasone and Droperidol each reduced the relative risk of PONV by ~25% and exhibited no synergistic interaction
- Propofol maintainance, when compared with volatile anaesthetic agents, reduced PONV by 19%
- Using oxygen and air as the carrier gas, when compared with oxygen and nitrous oxide, reduce PONV by 12%
- There was no statistically significant difference between 80% oxygen (PONV 31%) and 30% oxygen (PONV 24%)
- Using remifentanil, when compared with fentanyl, increased the risk of PONV by 5%, but this difference was not statistically significant
- Secondary outcome:
- Overall incidence of PONV
- Nausea: 31%
- Vomiting: 14%
- Nausea or Vomiting: 34%
- Median (mean) rating of worst nausea: 5 (5.7) on 11-point scale
- Median (mean) number of vomiting episodes: 1 (1.5)
- Adverse events
- Intra-operative vasopressors were required less often with propofol than with volatile anaesthetics
- Propofol: 15%
- Volatiles: 20%
- ARR: 5% (95% CI 2.8% to 7.2%; P < 0.001)
- NNT: 5
- Intra-operative vasopressors were required more often with remifentanil than with fentanyl
- Remifentanil: 21%
- Fentanyl: 13%
- ARI:7.1% (5.04% to 9.23%; P < 0.001)
- NNH: 15
- Post-operative shivering occurred more frequently with remifentanil than with fentanyl
- Remifentanil: 6.7%
- Fentanyl: 3.3%
- ARI: 3.4% (2.19% to 4.65%; P < 0.001)
- NNH: 30
- Intra-operative vasopressors were required less often with propofol than with volatile anaesthetics
- Interactions
- Through multivariate analysis, comparing interventions with other interventions and interventions with known baseline covariates, only one interaction was identified
- Droperidol reduced PONV in females (32% vs 43%; Odds Ratio 0.61; P < 0.001) but not males (16% vs 17%; Odds Ratio 1.04; P = 0.82)
- Through multivariate analysis, comparing interventions with other interventions and interventions with known baseline covariates, only one interaction was identified
- Overall incidence of PONV
Authors’ Conclusions
- Ondansetron, Dexamethasone and Droperidol equally and independently reduce the incidence of PONV with no synergistic effect
- Using Propofol rather than volatile anaesthetics and the avoidance of nitrous oxide also reduce the incidence of PONV but to a lesser extent
- Remifentanil offers no beneficial effect over fentanyl for PONV prevention
- A strategy involving risk assessment followed by layered, multi-model interventions with the cheapest and safest being used first is recommended
Strengths
- This is an important clinical question to research given the frequency of surgery worldwide and the incidence of PONV
- The ambitious multi-factorial design is an efficient method to answer several questions in one study
- Appropriate randomisation method and concealment of allocation sequence prior to patient recruitment
- Guidance for the anaesthetic care will reduce the possibility of influence from other factors but it was not possible to control every aspect
- Multi-centre recruitment and block randomisation (i.e. each centre had equal distribution of allocations) will reduce the likelihood of such influences producing a biased result
- Post-op care providers, patients and outcome assessors were blinded to allocation, which reduces maintenance, reporting and assessment bias
Weaknesses
- It could be argued that withholding all antiemetic interventions from the patients randomised to that allocation is unethical, given they were identified as high-risk and there are proven beneficial options
- The beta-error (power) of the study is not specified
- The anaesthetist providing care intra-operatively was aware of the group allocation (unblinded) and could have influenced the outcome consciously or unconsciously
- Given the number of centres and anaesthetists involved, this is unlikely to have systematically biased the overall results
- Given the high prevalence of female patients (by design as this is a risk factor), the results regarding males may be underpowered and it may not be possible to extrapolate the overall findings to males with the same confidence
- The finding that droperidol did not reduce PONV in men may be a chance finding with low power
- No sensitivity analysis investigated the potential impact of the 4.4% patients missing outcome data
- This is well below the primary outcome incidence so it is unlikely to have changed the primary outcomes
- The sampled population is all European and no data are presented for ethnicity
- It may not be possible to extrapolate the conclusion to non-European populations
- Safety data are limited in this trial, and some commentators have suggested the conclusion of “use the cheapest and safest” is difficult to draw from this trial alone
The Bottom Line
- This landmark trial provides good evidence to guide clinical practice and communication with patients
- Beneficial interventions include: ondansetron, dexamethasone, droperidol, total intravenous anaesthesia (TIVA) with propofol, and avoidance of nitrous oxide
- This trial did not include regional anaesthesia interventions with opiate-sparing effects, which warrant further investigations
External Links
- [article] A Factorial Trial of Six Interventions for the Prevention of Postoperative Nausea and Vomiting
- [further reading] Simplified algorithm for the prevention of postoperative nausea and vomiting: a before-and-after study
- [further reading] How to prevent and manage postoperative nausea and vomiting
- [further reading] Acupuncture in the prevention of postoperative nausea and vomiting
Metadata
Summary author: Duncan Chambler
Summary date: 20 October 2018
Peer-review editor: David Slessor



